Region-specific quantitation of glycosphingolipids in the elderly human brain with Nanoflow MEA Chip Q/ToF mass spectrometry
- PMID: 40207879
- PMCID: PMC12021261
- DOI: 10.1093/glycob/cwaf022
Region-specific quantitation of glycosphingolipids in the elderly human brain with Nanoflow MEA Chip Q/ToF mass spectrometry
Abstract
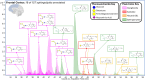
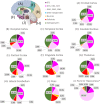
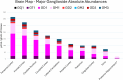
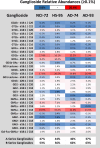
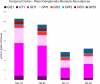
Glycosphingolipids are a unique class of bioactive lipids responsible for lateral membrane organization and signaling found in high abundance in the central nervous system. Using nanoflow MEA Chip Q/ToF mass spectrometry, we profiled the intact glycosphingolipids of the elderly human brain in a region-specific manner. By chromatographic separation of glycan and ceramide isomers, we determined gangliosides to be the highest source of heterogeneity between regions with the expression of a- and b-series glycan structures. Investigation of these trends showed that specific glycan structures were, in part, determined by the structure of their lipid backbone. This study provides insight into the dynamic process of membrane remodeling in the brain during aging.
Keywords: MEA Chip; Nanoflow HPLC-Q; ToF; brain map; glycosphingolipids.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures


References
-
- Boggs JM, Gao W, Hirahara Y. 2008. Myelin glycosphingolipids, Galactosylceramide and Sulfatide, participate in carbohydrate–carbohydrate interactions between apposed membranes and may form Glycosynapses between oligodendrocyte and/or myelin membranes. Biochim Biophys Acta (BBA). 1780:445–455. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

