Effect of urolithin A on intracellular survival of Mycobacterium tuberculosis by regulating AKT-FOXO1-mediated autophagy
- PMID: 40207917
- PMCID: PMC12108056
- DOI: 10.1128/msphere.00061-25
Effect of urolithin A on intracellular survival of Mycobacterium tuberculosis by regulating AKT-FOXO1-mediated autophagy
Abstract
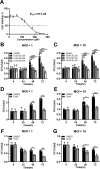
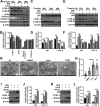
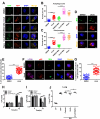
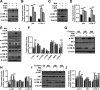
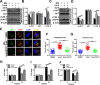
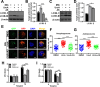
Tuberculosis (TB), resulting from Mycobacterium tuberculosis (Mtb), is one of the leading causes of morbidity and mortality in humans worldwide. Host-directed therapy (HDT) is a novel approach for treating TB, particularly those with drug resistance. Urolithin A (UroA) produced through bioconversion of plant-derived ellagic acid by gut microbes has been proven to have multiple beneficial effects in a variety of diseases without showing undesired adverse reactions. However, whether UroA has antimycobacterial effect and the underlying mechanism has not yet been reported. Here, we found that UroA significantly inhibited Mtb growth within both macrophages and mice. Moreover, UroA promoted the activation of autophagy in Mtb-infected macrophages via the protein kinase B-Forkhead box protein O1 signaling pathway, which contributed to the antimycobacterial effect of UroA. Additionally, UroA suppressed the survival of clinically isoniazid (INH)-resistant Mtb (C2) within macrophages, and the combination of UroA and INH synergistically enhanced host elimination of Mtb H37Rv. Therefore, UroA may be utilized as a potential candidate for HDT and as an adjunctive therapy with first-line anti-TB drugs.IMPORTANCEHost-directed therapy (HDT) is a novel approach for treating tuberculosis (TB), particularly those with drug resistance. Urolithin A (UroA) produced through bioconversion of plant-derived ellagic acid by gut microbes has been proven to have multiple beneficial effects in a variety of diseases without showing undesired adverse reactions. We found that UroA significantly inhibited Mycobacterium tuberculosis (Mtb) growth within macrophages. Moreover, UroA suppressed the survival of clinically isoniazid (INH)-resistant Mtb (C2) within macrophages, and the combination of UroA and INH synergistically enhanced host elimination of Mtb H37Rv. Therefore, UroA may be utilized as a potential candidate for HDT and as an adjunctive therapy with first-line anti-TB drugs.
Keywords: AKT-FOXO1 signaling pathway; HDT; Mycobacterium tuberculosis; autophagy; urolithin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Pasakbumin A controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and production of antibacterial mediators in mouse macrophages.PLoS One. 2019 Mar 13;14(3):e0199799. doi: 10.1371/journal.pone.0199799. eCollection 2019. PLoS One. 2019. PMID: 30865638 Free PMC article.
-
Macrophage targeted polymeric curcumin nanoparticles limit intracellular survival of Mycobacterium tuberculosis through induction of autophagy and augment anti-TB activity of isoniazid in RAW 264.7 macrophages.Front Immunol. 2023 Jul 31;14:1233630. doi: 10.3389/fimmu.2023.1233630. eCollection 2023. Front Immunol. 2023. PMID: 37583694 Free PMC article.
-
Host-directed therapy with amiodarone in preclinical models restricts mycobacterial infection and enhances autophagy.Microbiol Spectr. 2024 Aug 6;12(8):e0016724. doi: 10.1128/spectrum.00167-24. Epub 2024 Jun 25. Microbiol Spectr. 2024. PMID: 38916320 Free PMC article.
-
Nanomaterial-mediated host directed therapy of tuberculosis by manipulating macrophage autophagy.J Nanobiotechnology. 2024 Oct 8;22(1):608. doi: 10.1186/s12951-024-02875-w. J Nanobiotechnology. 2024. PMID: 39379986 Free PMC article. Review.
-
Host-directed therapy against mycobacterium tuberculosis infections with diabetes mellitus.Front Immunol. 2024 Jan 8;14:1305325. doi: 10.3389/fimmu.2023.1305325. eCollection 2023. Front Immunol. 2024. PMID: 38259491 Free PMC article. Review.
References
-
- World Health Organization . 2023. Global tuberculosis report 2023
-
- Bruns H, Stegelmann F, Fabri M, Döhner K, van Zandbergen G, Wagner M, Skinner M, Modlin RL, Stenger S. 2012. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol 189:4069–4078. doi:10.4049/jimmunol.1201538 - DOI - PMC - PubMed
-
- Jurado JO, Alvarez IB, Pasquinelli V, Martínez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE, García VE. 2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 181:116–125. doi:10.4049/jimmunol.181.1.116 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
