Persistence of CMV-specific anti-HIV CAR T cells after adoptive immunotherapy
- PMID: 40207929
- PMCID: PMC12090794
- DOI: 10.1128/jvi.01933-24
Persistence of CMV-specific anti-HIV CAR T cells after adoptive immunotherapy
Abstract
The success of chimeric antigen receptor (CAR)-T cell (Tc) immunotherapy in refractory B-cell acute lymphoblastic leukemia (B-ALL) suggests adaptation of this strategy toward HIV. Because cytomegalovirus (CMV) vaccine vectors generated Tc responses that controlled viral replication, these studies aim to genetically modify CMV-specific Tc with HIV-CAR2 vectors and link HIV immunotherapy to persistent CMV antigen stimulation. To mimic a clinical scenario, rhesus macaques were challenged with the CCR5-tropic simian/human immunodeficiency virus (SHIV-D) prior to antiretroviral therapy (ART). Autologous CMV-specific Tc were transduced with the control CEA-CAR2 or CD4-CAR2/maC46 vectors and reinfused. After stopping ART, the plasma viral load (PVL) in the control rebounded and was sustained above 1.7 × 104 copies/mL; PVL in CD4-CAR2-treated animals was delayed up to 6 weeks and 10-fold lower. The CD4 CAR-Tc frequency peaked at day 7 and was detected in lymphoid tissues at 6 weeks. Both CEA-CAR2 and CD4-CAR2 persisted in PBMCs for about 2 years, which indicates that the CMV-specific CAR Tc were maintained based on their CMV specificity. However, long-term PVL was stable in all animals. Thus, CMV-specific CAR-Tc were active initially, persisted long term, but failed to control viral replication.IMPORTANCEBecause of latent viral reservoirs and a dysfunctional immune response, HIV replication rebounds when antiretroviral therapy is interrupted. Therefore, cytomegalovirus (CMV)-specific Tc were genetically modified with anti-HIV CD4-CAR2 vectors to link the targeting of the HIV envelope to the persistent CMV immune response. In this clinical scenario with simian/human immunodeficiency virus (SHIV) challenge and antiretroviral therapy (ART) suppression, early activity of the CAR Tc delayed rebound in the rhesus macaque/SHIV challenge model. However, even with long-term persistence of CAR Tc in the blood, control of viral replication was not achieved. These data suggest that CAR Tc will require additional interventions to cure HIV infection.
Keywords: CD4-CAR T cells; CMV-specific immune responses; adoptive immunotherapy; persistence and biodistribution; rhesus SHIV-challenge model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
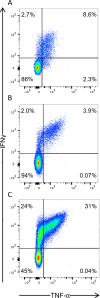

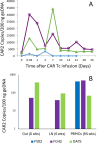
References
-
- Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J Virol 75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001 - DOI - PMC - PubMed
-
- Eller MA, Goonetilleke N, Tassaneetrithep B, Eller LA, Costanzo MC, Johnson S, Betts MR, Krebs SJ, Slike BM, Nitayaphan S, Rono K, Tovanabutra S, Maganga L, Kibuuka H, Jagodzinski L, Peel S, Rolland M, Marovich MA, Kim JH, Michael NL, Robb ML, Streeck H. 2016. Expansion of inefficient HIV-specific CD8 T cells during acute infection. J Virol 90:4005–4016. doi: 10.1128/JVI.02785-15 - DOI - PMC - PubMed
-
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD, Altfeld M. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med 200:701–712. doi: 10.1084/jem.20041270 - DOI - PMC - PubMed
-
- Ndhlovu ZM, Kamya P, Mewalal N, Kløverpris HN, Nkosi T, Pretorius K, Laher F, Ogunshola F, Chopera D, Shekhar K, Ghebremichael M, Ismail N, Moodley A, Malik A, Leslie A, Goulder PJR, Buus S, Chakraborty A, Dong K, Ndung’u T, Walker BD. 2015. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity 43:591–604. doi: 10.1016/j.immuni.2015.08.012 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R33 AI110158/AI/NIAID NIH HHS/United States
- AI110158/National Institute of Allergy and Infectious Diseases
- 75N92019D00018/HL/NHLBI NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- AI102847/National Institute of Allergy and Infectious Diseases
- Pilot/Tulane University School of Medicine
- F30 AI150452/AI/NIAID NIH HHS/United States
- F30AI150452/National Institute of Allergy and Infectious Diseases
- R56 AI102847/AI/NIAID NIH HHS/United States
- S10 OD026800/OD/NIH HHS/United States
- R44 AI152709/AI/NIAID NIH HHS/United States
- P51 OD011104/RR/NCRR NIH HHS/United States
- R21 AI110158/AI/NIAID NIH HHS/United States
- AI152709/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources
Medical
Research Materials

