The ACBD3 protein coordinates ER-Golgi contacts to enable productive TBEV infection
- PMID: 40207930
- PMCID: PMC12090792
- DOI: 10.1128/jvi.02224-24
The ACBD3 protein coordinates ER-Golgi contacts to enable productive TBEV infection
Abstract
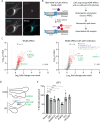
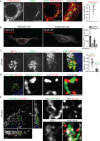
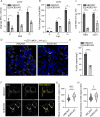
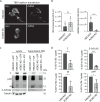
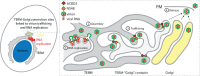
Flavivirus infection involves extensive remodeling of the endoplasmic reticulum (ER), which is key to both the replication of the viral RNA genome as well as the assembly and release of new virions. However, little is known about how viral proteins and host factors cooperatively facilitate such a vast transformation of the ER, and how this influences the different steps of the viral life cycle. In this study, we screened for host proteins that were enriched in close proximity to the tick-borne encephalitis virus (TBEV) protein NS4B and found that the top candidates were coupled to trafficking between ER exit sites (ERES) and the Golgi. We characterized the role of ACBD3, one of the identified proteins, and showed that it promotes TBEV infection. Depletion of ACBD3 inhibited virus replication and resulted in abnormal transformation of the ER, leading to reduced virion release. ACBD3's proviral mechanism did not involve the recruitment of PI4PK as previously described for enteroviruses. Instead, productive TBEV infection required the full-length ACBD3, which localizes to ER-Golgi contact sites together with NS4B. We propose that NS4B and ACBD3 promote replication by coordinating the transformation of the ER, which is required for RNA replication and particle release. The transformation involves direct coupling to the Golgi which facilitates efficient virion transport.
Importance: Flaviviruses like tick-borne encephalitis have significant effects on human health. During flavivirus infection, the viral particles enter the host cells and transform the endoplasmic reticulum (ER), which is a membranous organelle and the main site of cellular protein synthesis. Although this is critical for successful infection, the details of the process are unknown. Here, we found that the viral protein NS4B and the host protein ACBD facilitate this transformation by ensuring that the ER is coupled to the Golgi apparatus, the organelle responsible for transporting material out of the cell. TBEV uses ACBD3 to guarantee that the connection sites between the transformed ER and the Golgi remain functional so that RNA is replicated and the produced viral particles are exported from the cell and can infect further cells. Our work sheds light both on the basic biology of flavivirus infection, and virus-induced remodeling of membranous organelles.
Keywords: ACBD3; ER exit sites; ERES-Golgi contact; NS4B; Orthoflavivirus; flavivirus; host-pathogen interaction; replication organelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Postler TS, Beer M, Blitvich BJ, Bukh J, de Lamballerie X, Drexler JF, Imrie A, Kapoor A, Karganova GG, Lemey P, Lohmann V, Simmonds P, Smith DB, Stapleton JT, Kuhn JH. 2023. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol 168:224–227. doi:10.1007/s00705-023-05835-1 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

