Cell binding, uptake, and infection of influenza A virus using recombinant antibody-based receptors
- PMID: 40207931
- PMCID: PMC12090727
- DOI: 10.1128/jvi.02275-24
Cell binding, uptake, and infection of influenza A virus using recombinant antibody-based receptors
Abstract
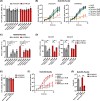
Human and avian influenza A viruses bind to sialic acid (Sia) receptors on cells as their primary receptors, and this results in endocytic uptake of the virus. While the role of Sia on glycoproteins and/or glycolipids for virus entry is crucial, the roles of the carrier proteins are still not well understood. Furthermore, it is still unclear how receptor binding leads to infection, including whether the receptor plays a structural or other roles beyond being a simple tether. To enable the investigation of the receptor binding and cell entry processes in a more controlled manner, we have designed a protein receptor for pandemic H1 influenza A viruses. The engineered receptor possesses the binding domains of an anti-HA antibody prepared as a single-chain variable fragment (scFv) fused with the stalk, transmembrane, and cytoplasmic sequences of the feline transferrin receptor type-1 (fTfR). When expressed in cells that lack efficient display of Sia due to a knockout of the Slc35A1 gene, which encodes for the solute carrier family 35 transporter (SLC35A1), the anti-H1 receptor was displayed on the cell surface, bound virus, or hemagglutinin proteins, and the virus was efficiently endocytosed into the cells. Infection occurred at similar levels to those seen after reintroducing Sia expression, and lower affinity receptor mutants displayed enhanced infections. Treatment with clathrin-mediated endocytosis (CME) inhibitors significantly reduced viral entry, indicating that virus rescue by the antibody-based receptor follows a similar internalization route as Sia-expressing cells.IMPORTANCEInfluenza A viruses primarily circulate among avian reservoir hosts but can also jump species, causing outbreaks in mammals, including humans. A key interaction of the viruses is with host cell sialic acids, which vary in chemical form, in their linkages within the oligosaccharide, and in their display on various surface glycoproteins or glycolipids with differing properties. Here, we report a new method for examining the processes of receptor binding and uptake into cells during influenza A virus infection, by use of an engineered HA-binding membrane glycoprotein, where antibody variable domains are used to bind the virus, and the transferrin receptor uptake structures mediate efficient entry. This will allow us to test and manipulate the processes of cell binding, entry, and infection.
Keywords: endocytosis; experimental tools; host-cell interactions; orthomyxovirus; receptor binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The C-type Lectin Langerin Functions as a Receptor for Attachment and Infectious Entry of Influenza A Virus.J Virol. 2015 Oct 14;90(1):206-21. doi: 10.1128/JVI.01447-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468543 Free PMC article.
-
Influenza A Virus Agnostic Receptor Tropism Revealed Using a Novel Biological System with Terminal Sialic Acid Knockout Cells.J Virol. 2022 Aug 10;96(15):e0041622. doi: 10.1128/jvi.00416-22. Epub 2022 Jul 18. J Virol. 2022. PMID: 35862707 Free PMC article.
-
Single Particle Analysis of H3N2 Influenza Entry Differentiates the Impact of the Sialic Acids (Neu5Ac and Neu5Gc) on Virus Binding and Membrane Fusion.J Virol. 2023 Mar 30;97(3):e0146322. doi: 10.1128/jvi.01463-22. Epub 2023 Feb 13. J Virol. 2023. PMID: 36779754 Free PMC article.
-
Receptor binding and pH stability - how influenza A virus hemagglutinin affects host-specific virus infection.Biochim Biophys Acta. 2014 Apr;1838(4):1153-68. doi: 10.1016/j.bbamem.2013.10.004. Epub 2013 Oct 24. Biochim Biophys Acta. 2014. PMID: 24161712 Review.
-
Receptor binding properties of the influenza virus hemagglutinin as a determinant of host range.Curr Top Microbiol Immunol. 2014;385:63-91. doi: 10.1007/82_2014_423. Curr Top Microbiol Immunol. 2014. PMID: 25078920 Review.
Cited by
-
Identification of SLC35A1 as an essential host factor for the transduction of multi-serotype recombinant adeno-associated virus (AAV) vectors.bioRxiv [Preprint]. 2024 Oct 17:2024.10.16.618764. doi: 10.1101/2024.10.16.618764. bioRxiv. 2024. Update in: mBio. 2025 Jan 8;16(1):e0326824. doi: 10.1128/mbio.03268-24. PMID: 39463973 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

