Transformation of Gardnerella vaginalis with a Bifidobacterium-Escherichia coli shuttle vector plasmid
- PMID: 40207948
- PMCID: PMC12054147
- DOI: 10.1128/spectrum.00481-25
Transformation of Gardnerella vaginalis with a Bifidobacterium-Escherichia coli shuttle vector plasmid
Abstract
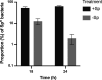
Gardnerella spp. significantly influence female reproductive health and are indicators of bacterial vaginosis, a common gynecological disorder. Lack of genetic tools for Gardnerella spp. is a hindrance to fully understanding their role in the vaginal microbiome, and no naturally occurring plasmids have yet been identified in these organisms. The aim of this study was to transform Gardnerella vaginalis and characterize transformants carrying Bifidobacterium-E. coli shuttle vector pKO403-lacZ'-Sp. G. vaginalis ATCC 49145 was selected for protocol development based on its high growth rate, lack of restriction activity, and susceptibility to spectinomycin. Low efficiency (~102 cfu/µg of plasmid DNA) but reproducible transformation was achieved. The expression of the spectinomycin resistance gene and the β-galactosidase gene of pKO403-lacZ'-Sp in G. vaginalis ATCC 49145 resulted in an increase in spectinomycin tolerance from 2 µg/mL (MIC) to >512 µg/mL, and an appreciable increase in β-galactosidase activity compared with the wild type. Plasmid copy number was determined to be ~3 per genome copy. Plasmid was lost rapidly in the absence of spectinomycin selection, with only ~5% of colony-forming units retaining the resistant phenotype after 24 h of growth without selection. These results demonstrate that G. vaginalis can be transformed by electroporation and that pKO403-lacZ'-Sp can be maintained and its genes expressed in this host, offering a starting point for the development of genetic tools for mechanistic studies of this important member of the vaginal microbiome.
Importance: The healthy human vaginal microbiome is mainly dominated by Lactobacillus spp. An imbalance or shift in this population can lead to a gynecological disorder known as bacterial vaginosis (BV). In BV, there is a reduction in Lactobacillus spp. and an overgrowth of mixed anaerobes and facultative bacteria including Gardnerella spp. The reason for this increase in the Gardnerella population and associated changes in the vaginal microbiota composition is yet not understood, and a lack of genetic tools is one of the major barriers to performing mechanistic research to study the biology of these clinically significant organisms. The first step in developing genetic tools is introducing foreign DNA. In this study, we have developed a protocol for transformation and identified a plasmid that can be maintained in G. vaginalis.
Keywords: Gardnerella; plasmid stability; plasmids; transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Greenwood JR, Pickett MJ. 1980. Transfer of Haemophilus vaginalis Gardner and Dukes to a new genus, Gardnerella: G. vaginalis (Gardner and Dukes) comb. nov. Int J Syst Bacteriol 30:170–178. doi: 10.1099/00207713-30-1-170 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

