Reliable high-PAP-1-loaded polymeric micelles for cancer therapy: preparation, characterization, and evaluation of anti-tumor efficacy
- PMID: 40207975
- PMCID: PMC11986873
- DOI: 10.1080/10717544.2025.2490269
Reliable high-PAP-1-loaded polymeric micelles for cancer therapy: preparation, characterization, and evaluation of anti-tumor efficacy
Abstract
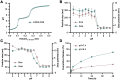
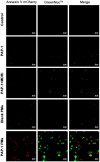
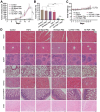
The mitochondrial potassium channel Kv1.3 is a critical therapeutic target, as its blockade induces cancer cell apoptosis, highlighting its therapeutic potential. PAP-1, a potent and selective membrane-permeant Kv1.3 inhibitor, faces solubility challenges affecting its bioavailability and antitumor efficacy. To circumvent these challenges, we developed a tumor-targeting drug delivery system by encapsulating PAP-1 within pH-responsive mPEG-PAE polymeric micelles. These self-assembled micelles exhibited high entrapment efficiency (91.35%) and drug loading level (8.30%). As pH decreased, the micelles exhibited a significant increase in particle size and zeta potential, accompanied by a surge in PAP-1 release. Molecular simulations revealed that PAE's tertiary amine protonation affected the self-assembly process, modifying hydrophobicity and resulting in larger, loosely packed particles. Furthermore, compared to free PAP-1 or PAP-1 combined with MDR inhibitors, PAP-1-loaded micelles significantly enhanced cytotoxicity and apoptosis induction in Jurkat and B16F10 cells, through mechanisms involving decreased mitochondrial membrane potential and elevated caspase-3 activity. In vivo, while free PAP-1 failed to reduce tumor size in a B16F10 melanoma mouse model, PAP-1-loaded micelles substantially suppressed tumors, reducing volume by up to 94.26%. Fluorescent-marked micelles effectively accumulated in mouse tumors, confirming their targeting efficiency. This strategy holds promise for significantly improving PAP-1's antitumor efficacy in tumor therapy.
Keywords: Mitochondrial potassium channel Kv1.3; PAP-1; anti-tumor; pH-responsive; polymeric micelles.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Abdul, M., Santo, A., and Hoosein, N., 2003. Activity of potassium channel-blockers in breast cancer. Anticancer research, 23 (4), 3347–3351. - PubMed
-
- Artym, V.V., and Petty, H.R., 2002. Molecular proximity of Kv1.3 voltage-gated potassium channels and beta(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. The journal of general physiology, 120 (1), 29–37. doi: 10.1085/jgp.20028607. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
