European Academy of Neurology (EAN)/European Federation of Autonomic Societies (EFAS)/International Neuro-Urology Society (INUS) Guidelines for Practising Neurologists on the Assessment and Treatment of Neurogenic Urinary and Sexual Symptoms (NEUROGED Guidelines)
- PMID: 40208234
- PMCID: PMC11984325
- DOI: 10.1111/ene.70119
European Academy of Neurology (EAN)/European Federation of Autonomic Societies (EFAS)/International Neuro-Urology Society (INUS) Guidelines for Practising Neurologists on the Assessment and Treatment of Neurogenic Urinary and Sexual Symptoms (NEUROGED Guidelines)
Erratum in
-
Correction to European Academy of Neurology (EAN)/European Federation of Autonomic Societies (EFAS)/International Neuro-Urology Society (INUS) Guidelines for Practising Neurologists on the Assessment and Treatment of Neurogenic Urinary and Sexual Symptoms (NEUROGED Guidelines).Eur J Neurol. 2025 May;32(5):e70205. doi: 10.1111/ene.70205. Eur J Neurol. 2025. PMID: 40364541 Free PMC article. No abstract available.
Abstract
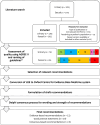
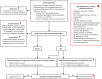
Background: Urinary and sexual symptoms are common following neurological disease, and we aimed to develop multidisciplinary inter-society evidence-based management guidelines.
Methods: The ADAPTE framework was used, and a systematic search of guidelines published in different languages was performed. Guidelines, consensus statements, and systematic reviews were included, and guideline quality was appraised using AGREE II. Patient representatives reviewed the relevance and suitability of recommendations. A modified Delphi process integrating the Evidence to Decision framework adapted from GRADE and the Oxford Centre for Evidence Based Medicine system was used to reach consensus on recommendation wording and strength.
Results: Recommendations were drafted, using guidelines/consensus statements (59 urinary, 50 sexual), systematic reviews (8 urinary, 2 sexual) and others (7 urinary,13 sexual), and wordings/strengths achieved at least 80% consensus through 2 Delphi rounds. Eleven evidence-based recommendations, 19 good practice statements, and 8 consensus-based recommendations were made. Individuals with neurological diseases should be asked about urogenital symptoms and undergo targeted physical examination when appropriate. Urinary symptom assessments include urinalysis, bladder diary completion, and post-void residual volume measurement. Treatments include fluid intake optimization, pelvic physiotherapy, tibial nerve stimulation, and oral medications. Urinary retention is managed by intermittent catheterization. Antibiotics should not be recommended to treat asymptomatic bacteriuria. Suprapubic catheterization is preferred for long-term catheterization. A comprehensive sexual history should be taken, focusing on multidimensional factors affecting sexual health. Treatments include lubricants, vibrators, and phosphodiesterase-5 inhibitors. Red flag symptoms warrant a shared-care approach with specialist colleagues.
Conclusions: The 38 NEUROGED recommendations will guide neurologists to comprehensively manage urogenital symptoms reported by individuals with neurological diseases.
Keywords: guideline; neurogenic; sexual dysfunction; treatment; urinary dysfunction; urogenital.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
J.N.P.: Consultant (Idorsia, Coloplast, Medice), Speaker Honorarium (Coloplast, Wellspect), Royalties (Cambridge University Press). A.F.: Royalties from Springer Verlag, speaker fees, and honoraria from Theravance Biopharma, GE Health Care, Bial, CNSystems, Broadview Ventures, KABEG, Austrian Autonomic Society, Elsevier, International Parkinson Disease and Movement Disorders Society, Austrian Neurology Society, Austrian Autonomic Society, and research grants from the FWF‐Austrian Science Fund, Medical University of Innsbruck, US MSA Coalition, Dr. Johannes and Hertha Tuba Foundation, and Austrian Exchange Program, outside of the present work. M.K.S.: Participated as a clinical investigator and/or received speaker fees fromSanofi Genzyme, Merck, Novartis, and Roche. I.A.: Participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Novartis, Roche, and AstraZeneca. M.A.A.: Consultant (Coloplast), speaker honorarium (Medtronic, Coloplast, GSK, Boston Scientific). J.M.P.: Speaker Honorarium (Asofarma, Convatec). A.B.: Has received a speaker honorarium from Ipsen Pharma and receives royalties from the book “Understanding Parkinsonism” (Jaypee brothers 2017). B.B.: Consultant (Coloplast), Speaker Honorarium (Coloplast), Research Grant (Axonics). C.H.: Consultant: Convatec, BBraun. Speaker: IPSEN, Abbvie, Hollister Inc., Convatec. K.P.S.N.: Has led clinical trials on spasticity in M.S. for Celgene and GWS pharma and received royalties from Cambridge University Press. M.S.: Honoraria from Abbvie. R.D.T.: Lecture and consultancy fees from Medtronic, UCB, Theravarance, LivAssured, Zogenix, Novartis, and Arvelle, and grants from Medtronic and NewLife Wearables. M.T.: Works as a DBS (deep brain stimulation) consultant for Medtronic. M.H.: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Novartis, Roche, Astra Zeneca, and received funding from the Croatian Science Foundation. T.K., K.A., P.G., N.C., F.L., S.S., I.S., S.W., M.J.H., T.M.K., H.M., K.R.N., A.P.L.T., M.P., G.P., M.P., R.S., U.S., W.S., K.I.T., D.B.V., and G.W.: None declared.
Figures

References
-
- Low V., Ben‐Shlomo Y., Coward E., Fletcher S., Walker R., and Clarke C. E., “Measuring the Burden and Mortality of Hospitalisation in Parkinson's Disease: A Cross‐Sectional Analysis of the English Hospital Episodes Statistics Database 2009–2013,” Parkinsonism & Related Disorders 21, no. 5 (2015): 449–454, 10.1016/j.parkreldis.2015.01.017. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

