Impact of Natalizumab on Productivity and Ability to Work in Patients with Multiple Sclerosis in France: The TITAN Study
- PMID: 40208417
- PMCID: PMC12089578
- DOI: 10.1007/s40120-025-00725-x
Impact of Natalizumab on Productivity and Ability to Work in Patients with Multiple Sclerosis in France: The TITAN Study
Abstract
Introduction: The TITAN study examined changes in productivity, ability to work, and quality of life (QoL) before and after treatment with the high-efficacy therapy natalizumab (TYSABRI®) in patients with multiple sclerosis (MS) in France.
Methods: Patients, aged ≥ 18 and < 65 years with relapsing-remitting MS, either naïve to natalizumab or with ≤ 1 prior natalizumab infusion, with paid employment, were evaluated for productivity (number of working hours) in the 12 months prior to and after natalizumab initiation. Changes in annualized relapse rate and Expanded Disability Status Scale (EDSS) score were assessed. Changes in work status, working ability, physical and psychologic functioning, and QoL were also evaluated.
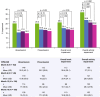
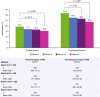
Results: Of 185 enrolled patients, the primary analysis population comprised 162 patients with a mean (SD) age of 36.8 (9.6) years and a baseline mean (SD) EDSS score of 1.9 (1.4). Annual mean (SD) productivity (n = 160) decreased from 1284.4 (503.3) h before natalizumab to 1208.0 (575.3) h (p = 0.05) in the year after natalizumab initiation. Significant improvement was seen in overall activity impairment at 6, 12, and 18 months of natalizumab treatment (p < 0.001). Decreases in annualized relapse rate (p < 0.0001) and EDSS score (p < 0.05) were observed during this period. In addition, treatment-related improvements were observed in presenteeism (reduced work efficiency), overall work impairment, and absenteeism (p < 0.05); significant improvements in psychological and physical impact (p ≤ 0.01) of MS were reported.
Conclusions: These findings suggest that early treatment with natalizumab may improve the work function of patients with MS, thereby decreasing the economic burden of the disease and improving patient quality of life.
Keywords: COVID-19; MSIS-29; Relapsing-remitting multiple sclerosis; WPAI-MS; Work productivity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Patrick Vermersch: personal compensation for consulting from AB Science, Biogen, BMS-Celgene, Imcyse, Merck, Novartis, Roche, Sanofi, and Teva. Honoraria and support for meetings from Biogen, Janssen, Merck, Novartis, Roche, and Sanofi. Research grants from Merck, Sanofi, and Roche. Arnaud Kwiatkowski: personal compensation for consulting from Biogen, Merck, Novartis. Honoraria from Biogen, Merck, Novartis, Roche, and Sanofi; for meetings from Biogen, Janssen, Novartis, Roche, and Sanofi; for participation in advisory board from Novartis. Alain Créange: grants or contracts from Alexion, Biogen, BMS, Merck, Novartis, and Roche. Honoraria and support for meetings from Alexion, Biogen, and Novartis. Payment for expert testimony from Merck. Marilyn Gros and Marta Ruiz: employees of and may hold stock and/or stock options in Biogen. Nathalie Texier, Jérôme de Sèze, Christine Lebrun-Frenay and Sophie Fantoni-Quinton have nothing to disclose. Ethical Approval: The TITAN study was approved by the French Consultative Committee on Data Processing in Health Research; approved by the French Expert Committee on Health Research, Studies, and Evaluations (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé [CCTIRS]); and authorized by the French Data Protection Commission. The study was conducted in accordance with local French laws, including the French Data Protection Act (Informatique et Libertés) and French Expert Committee on Health Research, Studies, and Evaluations (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé [CEREES]).
Figures


References
-
- Dobson R, Giovannoni G. Multiple sclerosis—a review. Eur J Neurol. 2019;26(1):27–40. - PubMed
-
- Lebrun-Frenay C, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for France. Mult Scler. 2017;23(2_suppl):65–77. - PubMed
-
- Chen J, Taylor BV, Blizzard L, et al. Effects of multiple sclerosis disease-modifying therapies on employment measures using patient-reported data. J Neurol Neurosurg Psychiatry. 2018;89(11):1200–7. - PubMed
LinkOut - more resources
Full Text Sources

