Spermidine toxicity in Saccharomyces cerevisiae due to mitochondrial complex III deficiency
- PMID: 40208436
- PMCID: PMC11985560
- DOI: 10.1007/s10522-025-10233-y
Spermidine toxicity in Saccharomyces cerevisiae due to mitochondrial complex III deficiency
Abstract
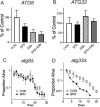
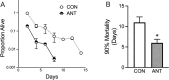
Spermidine is a naturally occurring polyamine present in all cells and is necessary for viability in eukaryotic cells. The cellular levels of spermidine decline as an organism ages, and its supplementation has been found to extend lifespan in yeast, worms, flies, mice, and human cultured cells. The lifespan extending effect of spermidine is thought to be due to its ability to induce autophagy, a turnover of cellular components. Mitochondrial dysfunction is believed to be a major driver of the aging process. We asked whether spermidine could rescue mitochondrial dysfunction using the yeast Saccharomyces cerevisiae lacking mtDNA (ρ0 cells) as a model. Not only was spermidine unable to rescue survival in ρ0 cells, but it appeared to exhibit toxicity resulting in a shortened lifespan. This toxicity appears to not be due to the loss of mitochondrial respiration, elevated oxidative stress, or depleted ATP. Spermidine toxicity could be recapitulated by the genetic or pharmacological inactivation of mitochondrial complex III. It can also be prevented by the impairment of autophagy, through the inactivation of ATG8, or by impairment of mitochondrial complex II through the inactivation of SDH2. Spermidine toxicity in ρ0 cells was present in yeast strains BY4741 and W303, but not D273-10B, demonstrating genetic variance in the phenotype. Thus, caution may be suggested regarding the use of spermidine to alleviate aging in humans. Depending on the genotype of the individual, spermidine could potentially harm the very individuals it is intended to help.
Keywords: Aging; Mitochondrial DNA; Mitochondrial dysfunction; Spermidine; Yeast.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
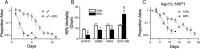

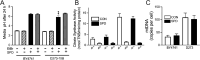


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

