Safety and Efficacy of Modular Digital Psychotherapy for Social Anxiety: Randomized Controlled Trial
- PMID: 40208666
- PMCID: PMC12022530
- DOI: 10.2196/64138
Safety and Efficacy of Modular Digital Psychotherapy for Social Anxiety: Randomized Controlled Trial
Abstract
Background: Social anxiety disorder is a common mental health condition characterized by an intense fear of social situations that can lead to significant impairment in daily life. Cognitive behavioral therapy (CBT) has been recognized as an effective treatment; however, access to therapists is limited, and the fear of interacting with therapists can delay treatment seeking. Furthermore, not all individuals respond. Tailoring modular treatments to individual cognitive profiles may improve efficacy. We developed a novel digital adaptation of CBT for social anxiety that is both modular and fully digital without a therapist in the loop and implemented it in the smartphone app Alena.
Objective: This study aimed to evaluate the safety, acceptability, and efficacy of the new treatment in online participants with symptoms of social anxiety.
Methods: In total, 2 web-based randomized controlled trials (RCTs) comparing individuals with access to the treatment through the app to a waitlist control group were conducted. Participants were recruited on the web and reported Social Phobia Inventory (SPIN) total scores of ≥30. Primary outcomes were safety and efficacy over 6 weeks in 102 women aged 18 to 35 years (RCT 1) and symptom reduction (SPIN scores) after 8 weeks in 248 men and women aged 18 to 75 years (RCT 2).
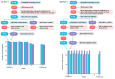
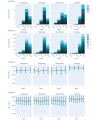
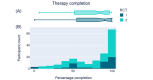
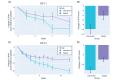
Results: In RCT 1, active and control arm adverse event frequency and severity were not distinguishable (intervention: 7/52, 13%; waitlist control: 8/50, 16%; χ21=0.007; P=.93). App acceptability was high, with a median completion rate of 90.91% (IQR 54.55%-100%). Secondary outcomes suggested greater symptom reduction in the active arm (mean SPIN score reduction -9.83, SD 12.80) than in the control arm (mean SPIN score reduction -4.13, SD 11.59; t90=-2.23; false discovery rate P=.04; Cohen d=0.47). RCT 2 replicated these findings. Adverse event frequency was comparable across the 2 groups (intervention: 20/124, 16.1%; waitlist control: 21/124, 16.8%; χ21<0.001; P>.99). Despite a longer treatment program, median completion remained high (84.85%, IQR 51.52%-96.97%). SPIN score reduction was greater in the active arm (mean -12.89, SD 13.87) than in the control arm (mean -7.48, SD 12.24; t227=-3.13; false discovery rate P=.008; Cohen d=0.42).
Conclusions: The web-only, modular social anxiety CBT program appeared safe, acceptable, and efficacious in 2 independent RCTs on online patient groups with self-reported symptoms of social anxiety.
Trial registration: ClinicalTrials.gov NCT05858294; https://clinicaltrials.gov/study/NCT05858294 (RCT 1) and ClinicalTrials.gov NCT05987969; https://clinicaltrials.gov/study/NCT05987969 (RCT 2).
Keywords: cognitive behavioral therapy; digital mental health; internet-delivered CBT; randomized controlled trial; social anxiety disorder.
©Mona M Garvert, Jessica McFadyen, Stuart Linke, Tayla McCloud, Sofie S Meyer, Sandra Sobanska, Paul B Sharp, Alex Long, Quentin J M Huys, Mandana Ahmadi. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 10.04.2025.
Conflict of interest statement
Conflicts of Interest: Authors MMG, JM, TM, SSM, SS, PBS, and AL were employed by Alena at the time of conducting this study, and SL was paid on a consultancy basis. MMG, JM, SSM, SS, AL, and MA own share options. QJMH is employed by University College London and acknowledges research grant funding from the Wellcome Trust, Carigest SA, and Koa Health and fees and share options for consultancies for Aya Technologies Ltd and Alto Neuroscience.
Figures


References
-
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994 Jan;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. - DOI - PubMed
-
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005 Jun;162(6):1179–87. doi: 10.1176/appi.ajp.162.6.1179. https://europepmc.org/abstract/MED/15930067 162/6/1179 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

