The PERK/ATF4 pathway is required for metabolic reprogramming and progressive lung fibrosis
- PMID: 40208691
- PMCID: PMC12128959
- DOI: 10.1172/jci.insight.189330
The PERK/ATF4 pathway is required for metabolic reprogramming and progressive lung fibrosis
Abstract
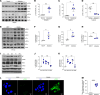
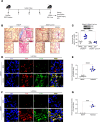
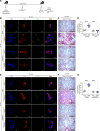
Asbestosis is a prototypical type of fibrosis that is progressive and does not resolve. ER stress is increased in multiple cell types that contribute to fibrosis; however, the mechanism(s) by which ER stress in lung macrophages contributes to fibrosis is poorly understood. Here, we show that ER stress resulted in protein kinase RNA-like ER kinase (PERK; Eif2ak3) activation in humans with asbestosis. Similar results were seen in asbestos-injured mice. Mice harboring a conditional deletion of Eif2ak3 were protected from fibrosis. Lung macrophages from asbestosis individuals had evidence of metabolic reprogramming to fatty acid oxidation (FAO). Eif2ak3fl/fl mice had increased oxygen consumption rate (OCR), whereas OCR in Eif2ak3-/- Lyz2-cre mice was reduced to control levels. PERK increased activating transcription factor 4 (Atf4) expression, and ATF4 bound to the Ppargc1a promoter to increase its expression. GSK2656157, a PERK-specific inhibitor, reduced FAO, Ppargc1a, and Aft4 in lung macrophages and reversed established fibrosis in mice. These observations suggest that PERK is a therapeutic target to reverse established fibrosis.
Keywords: Fatty acid oxidation; Fibrosis; Immunology; Macrophages; Pulmonology.
Figures





References
-
- Attfield MD, et al. Changing patterns of pneumoconiosis mortality--United States, 1968–2000. MMWR Morb Mortal Wkly Rep. 2004;53(28):627–632. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

