Efflux pumps and membrane permeability contribute to intrinsic antibiotic resistance in Mycobacterium abscessus
- PMID: 40208857
- PMCID: PMC12017575
- DOI: 10.1371/journal.ppat.1013027
Efflux pumps and membrane permeability contribute to intrinsic antibiotic resistance in Mycobacterium abscessus
Abstract
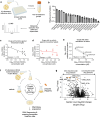
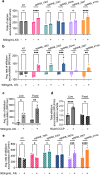
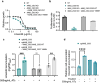
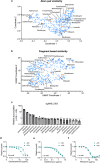
Mycobacterium abscessus is a pulmonary pathogen that exhibits intrinsic resistance to antibiotics, but the factors driving this resistance are incompletely understood. Insufficient intracellular drug accumulation could explain broad-spectrum resistance, but whether antibiotics fail to accumulate in M. abscessus and the mechanisms required for drug exclusion remain poorly understood. We measured antibiotic accumulation in M. abscessus using mass spectrometry and found a wide range of drug accumulation across clinically relevant antibiotics. Of these compounds, linezolid accumulates the least, suggesting that inadequate uptake impacts its efficacy. We utilized transposon mutagenesis screening to identify genes that cause linezolid resistance and found multiple transporters that promote membrane permeability or efflux, including an uncharacterized protein that effluxes linezolid and several chemically related antibiotics. This demonstrates that membrane permeability and drug efflux are critical mechanisms of antibiotic resistance in M. abscessus and suggests that targeting membrane transporters could potentiate the efficacy of certain antibiotics.
Copyright: © 2025 McGowen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Update of
-
Efflux pumps and membrane permeability contribute to intrinsic antibiotic resistance in Mycobacterium abscessus.bioRxiv [Preprint]. 2024 Aug 24:2024.08.23.609441. doi: 10.1101/2024.08.23.609441. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Apr 10;21(4):e1013027. doi: 10.1371/journal.ppat.1013027. PMID: 39229117 Free PMC article. Updated. Preprint.
References
-
- Nessar R, Cambau E, Reyrat J, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 2012;67(4):810–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

