A nonenzymatic dependency on inositol-requiring enzyme 1 controls cancer cell cycle progression and tumor growth
- PMID: 40208872
- PMCID: PMC12080931
- DOI: 10.1371/journal.pbio.3003086
A nonenzymatic dependency on inositol-requiring enzyme 1 controls cancer cell cycle progression and tumor growth
Abstract
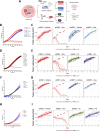
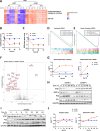
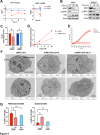
Endoplasmic-reticulum resident inositol-requiring enzyme 1α (IRE1) supports protein homeostasis via its cytoplasmic kinase-RNase module. Known cancer dependency on IRE1 entails its enzymatic activation of the transcription factor XBP1s and of regulated RNA decay. We discovered surprisingly that some cancer cell lines require IRE1 but not its enzymatic activity. IRE1 knockdown but not enzymatic IRE1 inhibition or XBP1 disruption attenuated cell cycle progression and tumor growth. IRE1 silencing led to activation of TP53 and CDKN1A/p21 in conjunction with increased DNA damage and chromosome instability, while decreasing heterochromatin as well as DNA and histone H3K9me3 methylation. Immunoelectron microscopy detected some endogenous IRE1 protein at the nuclear envelope. Thus, cancer cells co-opt IRE1 either enzymatically or nonenzymatically, which has significant implications for IRE1's biological role and therapeutic targeting.
Copyright: © 2025 Zuazo-Gaztelu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

