Downregulation of Nesprin1 by Runx2 deficiency is critical for the development of skeletal laminopathy-like pathology
- PMID: 40208950
- PMCID: PMC12012476
- DOI: 10.1073/pnas.2320138122
Downregulation of Nesprin1 by Runx2 deficiency is critical for the development of skeletal laminopathy-like pathology
Abstract
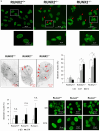
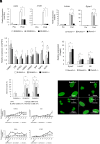
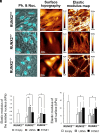
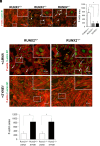
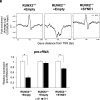
Runx2 is a master regulator of bone formation, and its dysfunction causes cleidocranial dysplasia (CCD) in humans. When iPS cells were generated from patients with CCD and Runx2-deficient iPS cells were generated using gene-editing techniques, abnormal laminopathy-like nuclei were observed. Runx2-deficient cells showed reduced Lamin A/C expression, but not protein levels. However, in Runx2-deficient cells, both the gene expression and protein levels of Nesprin1 were reduced, perinuclear actin fibers were sparser, and nuclear stiffness was reduced. Forced expression of Lamin A/C increased nuclear stiffness but did not improve nuclear morphology. In contrast, the induction of Nesprin1 expression alone normalized nuclear stiffness and restored nuclear morphology and perinuclear actin distribution. In Runx2-null cells, mechanical stress-induced phosphorylation of emerin was not observed. In contrast, forced expression of Nesprin1 in Runx2-null cells resulted in phosphorylation of emerin, indicating the restoration of intracellular tension. These observations were confirmed by atomic force microscopy. Therefore, the intracellular tension was inferred to pull the nuclear membrane into its normal shape. CUT&RUN assay and single RNA-seq analysis showed that an aberrant nuclear membrane caused loss of nuclear lamina gene regulation machinery, making the progression of normal osteogenic differentiation impossible; however, supplementation with Nesprin1 restored gene regulation mechanisms and promoted preosteoblast formation with normal nuclear morphology. Nesprin1 expression induced by Runx2 is essential for epigenetic regulation of the nuclear lamina. We propose CCD as a type of laminopathy involving defective expression of Nesprin1 regulated by Runx2.
Keywords: cleidocranial dysplasia; iPS cells; laminopathy; nuclear membrane; osteoblast differentiation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 19K10063/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H03833/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H03146/MEXT | Japan Society for the Promotion of Science (JSPS)
- MRCJD/Private University Research Branding Project from MEXT of Japan (Multidisciplinary Research Center for Jaw Disease (MRCJD): Achieving Longevity and Sustainability by Comprehensive Reconstruction of Or
LinkOut - more resources
Full Text Sources

