Formal Synthesis of Fostriecin via Asymmetric Alcohol-Mediated Carbonyl Allylation
- PMID: 40209063
- PMCID: PMC12288143
- DOI: 10.1021/acs.orglett.5c01026
Formal Synthesis of Fostriecin via Asymmetric Alcohol-Mediated Carbonyl Allylation
Abstract
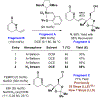
A formal synthesis of fostriecin via convergent assembly of two fragments prepared via asymmetric alcohol-mediated C-C coupling is described. One fragment is made by the enantioselective iridium-catalyzed allylation of an allylic alcohol mediated by allyl acetate. The other fragment is made via enantioselective ruthenium-catalyzed reductive syn-(α-alkoxy)allylation of an aldehyde mediated by an alkoxyallene (where 2-propanol is the hydrogen source), representing the first use of this method in target-oriented synthesis. Metathetic fragment union enables interception of a late-stage compound that previously required a 25 step (LLS) synthesis in only 7 steps (LLS).
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level via transfer hydrogenative coupling of allyl acetate: departure from chirally modified allyl metal reagents in carbonyl addition.J Am Chem Soc. 2008 Nov 5;130(44):14891-9. doi: 10.1021/ja805722e. Epub 2008 Oct 8. J Am Chem Soc. 2008. PMID: 18841896 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol or aldehyde oxidation level using allyl acetate as an allyl metal surrogate.J Am Chem Soc. 2008 May 21;130(20):6340-1. doi: 10.1021/ja802001b. Epub 2008 Apr 29. J Am Chem Soc. 2008. PMID: 18444616 Free PMC article.
-
Iridium-catalyzed anti-diastereo- and enantioselective carbonyl (trimethylsilyl)allylation from the alcohol or aldehyde oxidation level.J Am Chem Soc. 2010 Jul 7;132(26):9153-6. doi: 10.1021/ja103299f. J Am Chem Soc. 2010. PMID: 20540509 Free PMC article.
-
Enantioselective iridium-catalyzed carbonyl allylation from the alcohol oxidation level via transfer hydrogenation: minimizing pre-activation for synthetic efficiency.Chem Commun (Camb). 2009 Dec 21;(47):7278-87. doi: 10.1039/b917243m. Epub 2009 Oct 16. Chem Commun (Camb). 2009. PMID: 20024203 Free PMC article. Review.
-
Asymmetric Iridium-Catalyzed C-C Coupling of Chiral Diols via Site-Selective Redox-Triggered Carbonyl Addition.Top Curr Chem. 2016;372:85-101. doi: 10.1007/128_2015_651. Top Curr Chem. 2016. PMID: 26187028 Free PMC article. Review.
References
-
- Stampwala SS; Bunge RH; Hurley TR; Willmer NE; Brankiewicz AJ; Steinman CE; Smitka TA; French JC Novel Antitumor Agents CI-920, PD 113,270 and PD 113,271 II. Isolation and Characterization. J. Antibiot 1983, 36, 1601–1605. - PubMed
- Tunac JB; Graham BD; Dobson WE Novel Antitumor Agents CI-920, PD 113,270 and PD 113,271 I. Taxonomy, Fermentation, and Biological Properties. J. Antibiot 1983, 36, 1595–1600. - PubMed
-
- For isolation of fostriecin family of natural products, see: Ohkuma H; Naruse N; Nishiyama Y; Tsuno T; Hoshino Y; Sawada Y; Konishi M; Oki T Sultriecin A New Antifungal and Antitumor Antibiotic from Streptomyces roseiscleroticus: Production, Isolation, Structure and Biological Activity. J. Antibiot 1992, 45, 1239–1249. - PubMed
- Burke CP; Haq N; Boger DL Total Synthesis, Assignment of the Relative and Absolute Stereochemistry, and Structural Reassignment of Phostriecin (aka Sultriecin). J. Am. Chem. Soc 2010, 132, 2157–2159. - PMC - PubMed
- Burke CP; Swingle MR; Honkanen RE; Boger DL Total Synthesis and Evaluation of Phostriecin and Key Structural Analogues. J. Org. Chem 2010, 75, 7505–7513. - PMC - PubMed
- Amemiya M; Ueno M; Osono M; Masuda T; Kinoshita N; Nishida C; Hamada M; Ishizuka M; Takeuchi T Cytostatin, a Novel Inhibitor of Cell Adhesion to Components of Extracellular Matrix Produced by Streptomyces sp. MJ654-NF4 I. Taxonomy, Fermentation, Isolation, and Biological Activities. J. Antibiot 1994, 47, 536–540. - PubMed
- Amemiya M; Someno T; Sawa R; Naganawa H; Ishizuka M; Takeuchi T Cytostatin, a Novel Inhibitor of Cell Adhesion to Components of Extracellular Matrix Produced by Streptomyces sp. MJ654-NF4 II. Physico-chemical Properties and Structure Determination. J. Antibiot 1994, 47, 541–544. - PubMed
- Fushimi S; Nishikawa S; Shimazu A; Seto H Studies on New Phosphate Ester Antifungal Antibiotics Phoslactomycins I. Taxonomy, Fermentation, Purification, and Biological Activities. J. Antibiot 1989, 42, 1019–1025. - PubMed
- Fushimi S; Furihata K; Seto H Studies on New Phosphate Ester Antifungal Antibiotics Phoslactomycins II. Structure Elucidation of Phoslactomycins A to F. J. Antibiot 1989, 42, 1026–1036. - PubMed
- Shibata T; Kurihara S; Yoda K; Haruyama H Absolute Configuration of Leustroduscins. Tetrahedron, 1995, 51, 11999–12012.
- Thomasi SS; Ladeira C; Ferreira D; da Fontoura Sprenger R; Badino AC; Ferreira AG; Venâncio T Identification of Two New Phosphorylated Polyketides from a Brazilian Streptomyces sp. Through the Use of LC-SPE/NMR. Helv. Chim. Acta 2016. 99, 281–285.
-
- Boritzki TJ; Wolfard TS; Besserer JA; Jackson RC; Fry DW Inhibition of Type II Topoisomerase by Fostriecin. Biochem. Pharmacol 1988, 37, 4063–4068. - PubMed
-
- Roberge M; Tudan C; Hung SM; Harder KW; Jirik FR; Anderson H Antitumor Drug Fostriecin Inhibits the Mitotic Entry Checkpoint and Protein Phosphatases 1 and 2A. Cancer Res 1994, 54, 6115–6121. - PubMed
- Walsh AH; Cheng A; Honkanen RE Fostriecin, an Antitumor Antibiotic with Inhibitory Activity Against Serine/Threonine Protein Phosphatases Types 1 (PPl) and 2A (PP2A), is Highly Selective for PP2A. FEBS Lett. 1997, 416, 230–234. - PubMed
-
- Jastie CJ; Cohen PTW Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 1998, 431, 357–361. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

