Diagnosing Burkitt Lymphoma in Sub-Saharan Africa by Sequencing of Circulating Tumor DNA: A Comparative Microcosting Study
- PMID: 40209317
- PMCID: PMC12245731
- DOI: 10.1016/j.vhri.2025.101113
Diagnosing Burkitt Lymphoma in Sub-Saharan Africa by Sequencing of Circulating Tumor DNA: A Comparative Microcosting Study
Abstract
Objectives: Determining the cost of diagnosis of Burkitt lymphoma by DNA sequencing from a blood sample, compared with current histopathology. Estimating future sequencing costs at increased scale and exploring the effect of positivity rate on per-case cost.
Methods: We conducted a microcosting of both diagnostics. Resource use information was derived from standard operating procedures and interviews with staff. Unit cost data were from salary scales, purchase records, and publicly available prices. Costs were collected during 2021 and 2022, in the currency of purchase, and converted to common year (2024) and currency (US dollar [$]), with a discount rate of 5%. For increased scale, we assumed simple scaling up of current sample preparation and higher-capacity sequencing machines running at least once a week to maintain turnaround times.
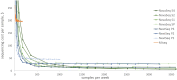
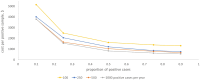
Results: We estimated a cost of $185.01 per patient for histopathology, with the main cost drivers being staining ($87.20, largely immunohistochemistry consumables, including $34.52 for antibodies) and the biopsy procedure ($72.29). The cost of the sequencing-based diagnostic was $710.15 at current throughput, with the largest contribution from the sequencing step because of the cost of sequencing reagents ($175.48 per sample). Costs are sensitive to throughput, reagent costs, and efficiency of utilization of equipment. At the current prevalence, cost per positive case is 2-fold higher at a positivity rate of 25% compared with 75%.
Conclusions: With the current technology and throughput, sequencing is likely to increase the cost of diagnosis compared with current pathology. Costs will reduce with increased scale, which requires establishing local reagent supply and maintenance capability.
Keywords: Burkitt lymphoma; circulating tumor DNA; diagnosis; microcosting; sub-Saharan Africa.
Copyright © 2025 International Society for Pharmacoeconomics and Outcomes Research, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author Disclosures Author disclosure forms can be accessed below in the Supplemental Material section. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK government.
Figures


References
-
- Crombie J., LaCasce A. The treatment of Burkitt lymphoma in adults. Blood. 2021;137(6):743–750. - PubMed
-
- Chantada G., Lam C.G., Howard S.C. Optimizing outcomes for children with non-Hodgkin lymphoma in low- and middle-income countries by early correct diagnosis, reducing toxic death and preventing abandonment. Br J Haematol. 2019;185(6):1125–1135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

