Treatment of Plaque Psoriasis with Guselkumab Reduces Systemic Inflammatory Burden as Measured by Neutrophil/Lymphocyte Ratio, Platelet/Lymphocyte Ratio, and Monocyte/Lymphocyte Ratio: A post hoc Analysis of Three Randomised Clinical Trials
- PMID: 40209685
- PMCID: PMC12143848
- DOI: 10.1159/000545148
Treatment of Plaque Psoriasis with Guselkumab Reduces Systemic Inflammatory Burden as Measured by Neutrophil/Lymphocyte Ratio, Platelet/Lymphocyte Ratio, and Monocyte/Lymphocyte Ratio: A post hoc Analysis of Three Randomised Clinical Trials
Abstract
Introduction: Psoriasis is associated with an increased risk of cardiovascular disease (CVD). Previous studies have found that treatment with tumour necrosis factor or interleukin (IL)-17 inhibitors leads to reductions in the systemic inflammation biomarkers neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and monocyte/lymphocyte ratio (MLR). The primary aim of this study was to evaluate changes in NLR, PLR and MLR with guselkumab compared with placebo (VOYAGE I/II), adalimumab (VOYAGE I/II), and secukinumab (ECLIPSE). The secondary aims were to assess correlation with disease severity, C-reactive protein (CRP) levels, and treatment response.
Methods: This was a post hoc analysis of VOYAGE I, VOYAGE II, and ECLIPSE Phase III randomised trial data on guselkumab for moderate-to-severe plaque psoriasis. NLR, PLR, and MLR were evaluated at baseline and Week 16 in VOYAGE I and II and at baseline and Week 12 in ECLIPSE. Mean changes were compared between groups using a Student's t test; Pearson's test was used for correlation analyses.
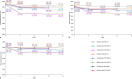
Results: VOYAGE I included 837 randomised patients, VOYAGE II included 992 randomised patients, and ECLIPSE included 1,048 randomised patients. In VOYAGE I, NLR (p = 0.011), PLR (p = 0.015), and MLR (p = 0.004) decreased significantly following 16 weeks of guselkumab treatment vs. placebo. In VOYAGE II, reductions in NLR (p = 0.003), PLR (p = 0.006), and MLR (p = 0.001) were greater at Week 16 in patients treated with guselkumab vs. placebo. Treatment with adalimumab was associated with a greater reduction (p < 0.001) in the three biomarkers vs. guselkumab, while secukinumab resulted in a similar reduction in NLR, PLR, and MLR compared with guselkumab (p = 0.413, 0.650, and 0.498, respectively). All biomarkers weakly correlated with Psoriasis Area and Severity Index (PASI) at baseline and showed modest correlations with CRP levels. Biomarkers in patients who were PASI90 responders were consistent between all active treatment groups at baseline.
Conclusions: Guselkumab is a highly efficacious treatment for plaque psoriasis; the study has demonstrated the potential benefit of treatment with guselkumab in reducing systemic inflammation as measured by NLR, PLR, and MLR, which appeared to be independent of psoriasis response, suggesting that reducing systemic inflammation with guselkumab may decrease CVD risk.
Keywords: Biologics; Biomarker; Guselkumab; Neutrophil-to-lymphocyte ratio; Platelet-to-lymphocyte ratio; Psoriasis; Systemic inflammation.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Niamh Kearney has received honoraria from AbbVie, Janssen, Lilly, Novartis, and UCB and has acted as a sub-investigator in clinical trials for AbbVie, MoonLake, and UCB. Patricia Gorecki was an employee of Janssen at the time this work was undertaken and is currently an employee of Kenvue, a Johnson & Johnson partner company. Lorenzo Acciarri is an employee of Valos, a Johnson & Johnson partner company. Jozefien Buyze is an employee of Janssen. Alianu Akawung is an employee of Janssen. Joseph F. Merola has acted as a consultant or investigator for AbbVie, Arena, Avotres, Biogen, Celgene, Dermavant, Lilly, EMD Serono, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharma, and UCB. Brian Kirby has received research support from or was a principal investigator in clinical trials for AbbVie, Almirall, Janssen, Merck Sharpe & Dohme, MoonLake, Novartis, Pfizer, and UCB; been a consultant for AbbVie, Almirall, Celgene, Janssen, Merck Sharpe & Dohme, MoonLake, Novartis, Pfizer, and UCB; received honoraria from AbbVie, Almirall, Celgene, Janssen, Lilly, MC2 Therapeutics, MoonLake, Novartis, Pfizer, and UCB; been on scientific advisory boards for AbbVie, Almirall, Celgene, GSK, Janssen, Lilly, MC2 Therapeutics, MoonLake, Novartis, Pfizer and UCB; was a member of the journal’s Editorial Board at the time of submission.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

