Clinical and Immunologic Impact of CMV Coinfection Among Children Living With HIV in Canada
- PMID: 40209769
- PMCID: PMC12240138
- DOI: 10.1097/INF.0000000000004811
Clinical and Immunologic Impact of CMV Coinfection Among Children Living With HIV in Canada
Abstract
Background: Although cytomegalovirus (CMV) disease has been well described among severely immunocompromised children living with HIV (CLWH), the impact of CMV coinfection, is not well understood. The objective of this study was to characterize the clinical and immunologic effects of CMV coinfection in CLWH in Canada.
Methods: This is a substudy of the Early Pediatric Initiation, Canada Child Cure Cohort study, which enrolled CLWH in Canada between 2014 and 2018. CMV serostatus was determined at the first (baseline) study visit, and HIV-1 viral load (VL), CMV VL, and lymphocyte subsets were quantified every 3-6 months. For a subset of participants, CD4 + and CD8 + T cell subsets were analyzed using flow cytometry. The clinical outcomes were recorded retrospectively at the baseline visit and prospectively during the study period.
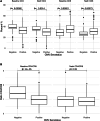
Results: Of the 225 participants, 85.3% were CMV seropositive (CMV + ) and 81% had suppressed HIV VL. While there were no significant differences in clinical outcomes between CMV + and CMV - children, CMV + children had lower frequencies of CD4 + T cells, higher frequencies of CD8 + T cells, and lower CD4/CD8 ratio at baseline than CMV - children. Children with CMV + children also demonstrated a higher frequency of CD4 + effector memory cells, lower CD8 + naïve T cells, and higher frequencies of CD8 + terminally differentiated effector memory cells. These differences remained significant even after adjusting for HIV viral control.
Conclusions: CMV coinfection is common among CLWH and is associated with distinct immunological changes despite the effective control of HIV replication with antiretroviral therapy. The long-term implications of these immunologic perturbations require further investigation.
Keywords: HIV; T cell subsets; children; coinfection; cytomegalovirus.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The CMV PCR testing kits and the AltoStar platform were donated by Altona Diagnostics. The authors declare the following conflicts: S.G.: consulting fees and/or research support from Moderna, Merck, GSK, VBI, Curevo and Altona. F.K.: research support from Altona, and Honoraria from ViV. The remaining authors declare no conflicts of interest. The funders had no role in the study design; collection, analyses or interpretation of data; writing of the manuscript or decision to publish the results.
Figures
References
-
- NIH, Centers for Disease Control and Prevention, HIV Medicine Association of the IDSA. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. 2023. Available at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult.... Accessed January 20, 2025.
-
- Nelson M, Dockrell D, Edwards S, et al. ; BHIVA Guidelines Subcommittee. British HIV Association and British Infection Association guidelines for the treatment of opportunistic infection in HIV-seropositive individuals 2011. HIV Med. 2011;12:1–140. Available at: https://www.bhiva.org/file/SwhaEzgXmAGOt/hiv_v12_is2_Iss2Press_Text.pdf. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials