Prevalence of ventricular parasystole in patients with cardiac sarcoidosis: correlation between parasystole and inflammation in ventricular fibrillation
- PMID: 40210253
- PMCID: PMC11987139
- DOI: 10.1136/openhrt-2025-003196
Prevalence of ventricular parasystole in patients with cardiac sarcoidosis: correlation between parasystole and inflammation in ventricular fibrillation
Abstract
Background: Ventricular parasystole is strongly associated with ventricular fibrillation (VF) in patients with non-ischaemic cardiomyopathy. However, the relationship between ventricular parasystole and cardiac sarcoidosis (CS) remains unclear. The purpose of this study was to examine the prevalence of parasystole in patients with CS.
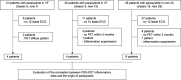
Methods: This was a retrospective observational study of 214 consecutive patients diagnosed with CS (mean age: 69±12 years, 104 males, median follow-up period: 6.8 years (IQR: 3.2-10.7) in our centre. We investigated parasystole in the patients who developed ventricular arrhythmia (VA) using 9886 ECGs, 280 Holter ECGs and 6391 implantable cardioverter defibrillator interrogation records. Classic parasystole was defined as three ventricular ectopic beats with the same morphology, occurring at integer-multiple intervals but with different coupling intervals (CI) on ECG. New parasystole was defined as two ventricular ectopic beats with a CI difference of more than 120 ms. We also analysed the correlation between inflammation sites and parasystole morphology observed on a 12-lead ECG.
Results: VA was identified in 95 patients (33.7%), and 22 developed VF (23.2%). Parasystole was observed in 12 of the 22 patients with VF (classic: 5, new: 7), 20 of 73 with ventricular tachycardia (classic: 5, new: 15) and 44 of 118 without VA (classic: 16, new: 28). Parasystole was significantly more common in the VF group than in the non-VF group (p=0.049). The site of inflammation observed on 18F-fluorodeoxyglucose positron emission tomography performed within 3 months after the development of VA and the origin of parasystole matched in all four patients with VF who had 12-lead ECG records of parasystole. Inflammation was correlated with the origin of parasystole.
Conclusion: Ventricular parasystole was detected in one-third of patients with CS in this study, especially those with VF. The presence of parasystole and inflammation may predict the occurrence of VF in patients with CS.
Keywords: Inflammation; Myocarditis; Tachycardia, Ventricular; Ventricular Fibrillation; Ventricular Premature Complexes.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: KK received speaker honoraria from Daiichi Sankyo Company, and Medtronic Japan, and research grants from Medtronic Japan and JSR. KM received funding/grants from Medtronic, Biosense Webster, Abbott and Boston and honoraria/speakers’ bureaus from Medtronic, Biosense Webster, Abbott and Boston outside the submitted work, and is affiliated with a department endowed by Medtronic outside the submitted work.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
