Elexacaftor/tezacaftor/ivacaftor in children aged ≥6 years with cystic fibrosis heterozygous for F508del and a minimal function mutation: results from a 96-week open-label extension study
- PMID: 40210412
- PMCID: PMC12256806
- DOI: 10.1183/13993003.02435-2024
Elexacaftor/tezacaftor/ivacaftor in children aged ≥6 years with cystic fibrosis heterozygous for F508del and a minimal function mutation: results from a 96-week open-label extension study
Abstract
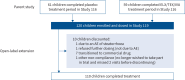
Background: Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was efficacious and safe in children aged 6-11 years with cystic fibrosis (CF) heterozygous for F508del and a minimal function CF transmembrane conductance regulator (CFTR) variant (F/MF genotypes) in a 24-week, placebo-controlled trial. We conducted a 96-week open-label extension study for children who completed the 24-week parent study.
Methods: In this phase 3b extension study, dosing was based on weight and age, with children weighing <30 kg and aged <12 years receiving ELX 100 mg once daily, TEZ 50 mg once daily and IVA 75 mg every 12 h, and children ≥30 kg or ≥12 years receiving ELX 200 mg once daily, TEZ 100 mg once daily and IVA 150 mg every 12 h. The primary end-point was safety and tolerability. Secondary and other efficacy end-points included absolute changes from parent study baseline in sweat chloride concentration, lung clearance index (LCI2.5), percentage predicted forced expiratory volume in 1 s (FEV1) and Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score.
Results: A total of 120 children were enrolled and dosed. 118 children (98.3%) had adverse events (AEs), which for most were mild (43.3%) or moderate (48.3%) in severity. The most common AEs (≥20% of children) were COVID-19 (58.3%), cough (51.7%), nasopharyngitis (45.0%), pyrexia (40.0%), headache (37.5%), upper respiratory tract infection (30.8%), oropharyngeal pain (26.7%), rhinitis (24.2%), abdominal pain (22.5%) and vomiting (20.0%). Children who transitioned from the placebo and ELX/TEZ/IVA groups of the parent study had improvements from parent study baseline at Week 96 in mean sweat chloride concentration (-57.3 (95% CI -61.6- -52.9) and -57.5 (95% CI -62.0- -53.0) mmol·L-1), LCI2.5 (-1.74 (95% CI -2.09- -1.38) and -2.35 (95% CI -2.72- -1.97) units), FEV1 % pred (6.1 (95% CI 2.6-9.7) and 6.9 (95% CI 3.2-10.5) percentage points) and CFQ-R respiratory domain score (6.6 (95% CI 2.5-10.8) and 2.6 (95% CI -1.6-6.8) points).
Conclusions: ELX/TEZ/IVA treatment was generally safe and well tolerated, with a safety profile consistent with the parent study and older age groups. After starting ELX/TEZ/IVA, children had robust improvements in sweat chloride concentration and lung function that were maintained through 96 weeks. These results demonstrate the safety and durable efficacy of ELX/TEZ/IVA in this paediatric population.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: M.A. Mall reports grants from Vertex Pharmaceuticals Incorporated, Boehringer Ingelheim, German Innovation Fund, German Ministry for Education and Research (BMBF) and German Research Foundation (DFG), travel support from Vertex Pharmaceuticals Incorporated and Boehringer Ingelheim, consulting fees from AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Splisense and Vertex Pharmaceuticals Incorporated, is a Fellow of the European Respiratory Society (FERS), and serves on the advisory board for AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Pari and Vertex Pharmaceuticals Incorporated. C.E. Wainwright received honoraria from Vertex Pharmaceuticals Incorporated and personal fees on a per-participant basis to institutions derived from pharmaceutical studies conducted, and served as Deputy Editor for Thorax and Associate Editor for Respirology, and on the advisory board to Vertex Pharmaceuticals Incorporated. J. Legg reports payment or honoraria for lectures, presentations, manuscript writing or educational events from the Vertex Pharmaceuticals Incorporated cystic fibrosis advisory board. M. Chilvers reports support for the current manuscript, consulting fees and payment or honoraria for educational events from Vertex Pharmaceuticals Incorporated, and serving as Chair of the Health Care Advisory Committee for Cystic Fibrosis Canada and Chair of the CF Working Group for the Canadian Thoracic Society. S. Gartner reports honoraria for lectures from Vertex Pharmaceuticals Incorporated. A-M. Dittrich reports grants or funding from Vertex Pharmaceuticals Incorporated (for execution of clinical studies) and German Federal Ministry of Education and Research (German Lung Center (DZL); for clinical research), payment or honoraria from Vertex Pharmaceutical Incorporated (for an article on the long-term effects of CFTR modulators and an online education forum Coliquio), StreamedUP (for an online educational presentation) and the Christiane Herzog Foundation (for a medical book on CF care), payment for expert testimony from the Austrian Society for Rare Disease, and leadership or fiduciary roles as a member of the German CF patient advocacy board, member of the German CF Clinical Trial Network Executive Committee, and as part of the ECFS CTN Executive Committee. F. Stehling reports clinical trial sponsorship and honoraria for lectures from Vertex Pharmaceuticals Incorporated. S. Connor, S. Grant, N. Suresh and T.G. Weinstock are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. J.C. Davies reports research grants from UK Cystic Fibrosis Trust, US CF Foundation, Cystic Fibrosis Ireland and EPSRC, clinical trial leadership and/or advisory board and speaking roles with Vertex Pharmaceuticals Incorporated, Boehringer Ingelheim, Eloxx, AlgiPharma, AbbVie, Arcturus, Enterprise Therapeutics, Recode, LifeArc, Genentech and Tavanta, and is Deputy Editor for the Journal of Cystic Fibrosis and President of the European Cystic Fibrosis Society.
Figures


Comment in
-
Transforming cystic fibrosis care: the impact of elexacaftor/tezacaftor/ivacaftor.Eur Respir J. 2025 Jul 14;66(1):2500793. doi: 10.1183/13993003.00793-2025. Print 2025 Jul. Eur Respir J. 2025. PMID: 40659466 No abstract available.
Similar articles
-
Long-Term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged ⩾6 Years with Cystic Fibrosis and at Least One F508del Allele: A Phase 3, Open-Label Clinical Trial.Am J Respir Crit Care Med. 2023 Jul 1;208(1):68-78. doi: 10.1164/rccm.202301-0021OC. Am J Respir Crit Care Med. 2023. PMID: 37154609 Free PMC article.
-
Vanzacaftor-tezacaftor-deutivacaftor versus elexacaftor-tezacaftor-ivacaftor in individuals with cystic fibrosis aged 12 years and older (SKYLINE Trials VX20-121-102 and VX20-121-103): results from two randomised, active-controlled, phase 3 trials.Lancet Respir Med. 2025 Mar;13(3):256-271. doi: 10.1016/S2213-2600(24)00411-9. Epub 2025 Jan 2. Lancet Respir Med. 2025. PMID: 39756424 Free PMC article. Clinical Trial.
-
Evaluation of elexacaftor-tezacaftor-ivacaftor treatment in individuals with cystic fibrosis and CFTRN1303K in the USA: a prospective, multicentre, open-label, single-arm trial.Lancet Respir Med. 2024 Dec;12(12):947-957. doi: 10.1016/S2213-2600(24)00205-4. Epub 2024 Aug 26. Lancet Respir Med. 2024. PMID: 39208836
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. doi: 10.1002/14651858.CD010966.pub4. Cochrane Database Syst Rev. 2023. PMID: 37983082 Free PMC article.
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
Cited by
-
Reported Adverse Events in Patients with CF Receiving Treatment with Elexacaftor/Tezacaftor/Ivacaftor: 5 Years Observational Study.J Clin Med. 2025 Jun 18;14(12):4335. doi: 10.3390/jcm14124335. J Clin Med. 2025. PMID: 40566080 Free PMC article.
References
-
- Cystic Fibrosis Foundation . About Cystic Fibrosis. 2024. www.cff.org/intro-cf/about-cystic-fibrosis Date last accessed: 13 March 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
