CDK4 loss-of-function mutations cause microcephaly and short stature
- PMID: 40210435
- PMCID: PMC7617628
- DOI: 10.1101/gad.352311.124
CDK4 loss-of-function mutations cause microcephaly and short stature
Abstract
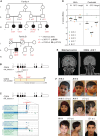
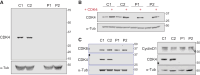
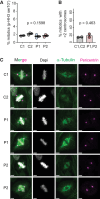
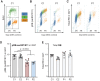
Cell number is a major determinant of organism size in mammals. In humans, gene mutations in cell cycle components result in restricted growth through reduced cell numbers. Here we identified biallelic mutations in CDK4 as a cause of microcephaly and short stature. CDK4 encodes a key cell cycle kinase that associates with D-type cyclins during G1 of the cell cycle to promote S-phase entry and cell proliferation through retinoblastoma (RB) phosphorylation. CDK4 and CDK6 are believed to be functionally redundant and are targeted jointly by chemotherapeutic CDK4/6 inhibitors. Using molecular and cell biology approaches, we show that functional CDK4 protein is not detectable in cells with CDK4 mutations. Cells display impaired RB phosphorylation in G1, leading to G1/S-phase transition defects and reduced cell proliferation, consistent with complete loss of cellular CDK4 enzymatic activity. Together, these findings demonstrate that CDK4 is itself required for cell proliferation, human growth, and brain size determination during development.
Keywords: cell cycle; centrosome; cyclin-dependent kinase; microcephalic dwarfism; microcephaly.
© 2025 Verdu Schlie et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
