Choice of colon capsule or colonoscopy versus default colonoscopy in FIT positive patients in the Danish screening programme: a parallel group randomised controlled trial
- PMID: 40210462
- PMCID: PMC12505048
- DOI: 10.1136/gutjnl-2024-333687
Choice of colon capsule or colonoscopy versus default colonoscopy in FIT positive patients in the Danish screening programme: a parallel group randomised controlled trial
Abstract
Background: Colonoscopy is among the standard tests for colorectal cancer (CRC) screening. However, uptake varies, and alternatives such as colon capsule endoscopy (CCE) are available. The uptake and detection rate of clinically significant neoplasia with CCE, compared with colonoscopy, remain unclear in this setting.
Objective: The primary objective of this study was to compare the detection rates of advanced neoplasia between CCE and colonoscopy, using a pathway in which the study group could choose between the two procedures, while the control group was offered only colonoscopy.
Design: A randomised, intention-to-treat trial was conducted among Danish CRC screening participants who tested positive with a faecal immunochemical test (FIT). The trial compared the detection rate of advanced neoplasia (primary outcome) and the uptake rate of both approaches between the two arms.
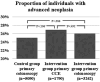
Results: A total of 473 684 invitations were sent to 396 676 individuals, with 62.6% returning the test. Among them, 11 075 tests were positive (4.5%), with no significant differences between the two study groups. Among FIT-positive cases, the uptake for colonoscopy was 91.1% in the control arm and 91.7% in the study arm, where participants had a choice of methods. In the study arm, 45.8% preferred CCE, 11.4% preferred colonoscopy and 42.8% had no preference and underwent colonoscopy. Ultimately, 69.9% of patients who initially opted for CCE were later referred for colonoscopy. The rate of advanced neoplasia detection was similar between the groups: 0.67% in the study arm versus 0.64% in the control arm.
Conclusion: Offering CCE as an alternative to colonoscopy did not significantly alter the detection rate of advanced neoplasia, nor did it increase uptake in a screening programme with high adherence to colonoscopy following a positive FIT test. Instead, it led to a very high rate of secondary colonoscopies. Therefore, CCE cannot be recommended in this setting.
Trial registration number: NCT04049357 (ClinicalTrials.gov).
Keywords: COLORECTAL ADENOMAS; COLORECTAL CANCER; DIAGNOSTIC AND THERAPEUTIC ENDOSCOPY; SCREENING.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form. GB was the main applicant for all funding stated for this manuscript and is a co-founder and co-owner of Stratos AI Aps. BS-O has received payments from Jinshan. AK has received payments from Jinshan, Medtronic, Covidien and Norgine. UD has received payment from Norgine. All other authors declare: no support from any organisation for the submitted work, and no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
