Transmembrane mucins in lung adenocarcinoma: understanding of current molecular mechanisms and clinical applications
- PMID: 40210618
- PMCID: PMC11985918
- DOI: 10.1038/s41420-025-02455-3
Transmembrane mucins in lung adenocarcinoma: understanding of current molecular mechanisms and clinical applications
Abstract
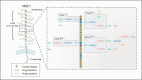
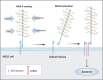
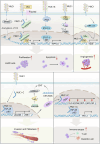
The mucin family is a group of highly glycosylated macromolecules widely present in human epithelial cells and with subtypes of secreted and membrane-associated forms. The membrane-associated mucins, known as transmembrane mucins, are not only involved in the formation of mucus barrier but also regulate cell signal transduction in physiological and pathological status. Transmembrane mucins could contribute to lung adenocarcinoma (LUAD) proliferation, apoptosis, angiogenesis, invasion, and metastasis, and remodel the immune microenvironment involved in immune escape. Furthermore, transmembrane mucins have been explored as potential LUAD indicators for diagnosis and prognosis. The development of targeted therapy and immunotherapeutic drugs targeting transmembrane mucins has also provided broad application prospects for clinic. In the following review, we summarize the characteristic structures of diverse transmembrane mucins, regulatory roles in promoting the progression of LUAD, and the current situation of diagnosis, prognosis, and therapeutic strategies based on transmembrane mucins.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This review article is a comprehensive analysis of existing scientific literature and does not involve human participants, animals, or the collection of new clinical data. As such, ethical approval or an ethics committee review was not required for this study.
Figures
References
-
- Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, Kaur S, et al. Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol. 2015;10:19–27. - PubMed
-
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

