Molecular basis for the phosphorylation of bacterial tyrosine kinase Wzc
- PMID: 40210632
- PMCID: PMC11986026
- DOI: 10.1038/s41467-025-58693-7
Molecular basis for the phosphorylation of bacterial tyrosine kinase Wzc
Abstract
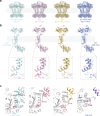
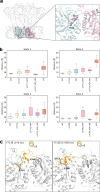
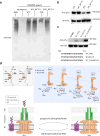
The regulation of polymerisation and translocation of biomolecules is fundamental. Wzc, an integral cytoplasmic membrane tyrosine autokinase protein serves as the master regulator of the biosynthesis and export of many bacterial capsular polysaccharides and exopolysaccharides. Such polysaccharides play essential roles in infection, defence, and some are important industrial products. Wzc comprises a large periplasmic domain, two transmembrane helices and a C-terminal cytoplasmic kinase domain with a tyrosine-rich tail. Wzc regulates polymerisation functions through cycling the formation and dissociation of an octameric complex, driven by changes in the phosphorylation status of the tyrosine-rich tail. E. coli Wzc serves a model for a wider family of polysaccharide co-polymerases. Here, we determine structures of intermediate states with different extents of phosphorylation. Structural and computational data reveal the pre-ordering of the tyrosine-rich tail, the molecular basis underlying the unidirectionality of phosphorylation events, and the underlying structural dynamics on how phosphorylation status is transmitted.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Whitfield, C., Wear, S. S. & Sande, C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol.74, 521–543 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

