An unconventional autophagic pathway that inhibits ATP secretion during apoptotic cell death
- PMID: 40210636
- PMCID: PMC11986000
- DOI: 10.1038/s41467-025-58619-3
An unconventional autophagic pathway that inhibits ATP secretion during apoptotic cell death
Abstract
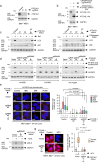
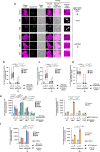
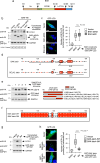
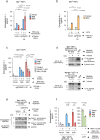
Mobilisation of Damage-Associated Molecular Patterns (DAMPs) determines the immunogenic properties of apoptosis, but the mechanisms that control DAMP exposure are still unclear. Here we describe an unconventional autophagic pathway that inhibits the release of ATP, a critical DAMP in immunogenic apoptosis, from dying cells. Mitochondrial BAK activated by BH3-only molecules interacts with prohibitins and stomatin-1 through its latch domain, indicating the existence of an interactome specifically assembled by unfolded BAK. This complex engages the WD40 domain of the autophagic effector ATG16L1 to induce unconventional autophagy, and the resulting LC3-positive vesicles contain ATP. Functional interference with the pathway increases ATP release during cell death, reduces ATP levels remaining in the apoptotic bodies, and improves phagocyte activation. These results reveal that an unconventional component of the autophagic burst that often accompanies apoptosis sequesters intracellular ATP to prevent its release, thus favouring the immunosilent nature of apoptotic cell death.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article.
-
Structural basis of BAK activation in mitochondrial apoptosis initiation.Nat Commun. 2022 Jan 11;13(1):250. doi: 10.1038/s41467-021-27851-y. Nat Commun. 2022. PMID: 35017502 Free PMC article.
-
In non-transformed cells Bak activates upon loss of anti-apoptotic Bcl-XL and Mcl-1 but in the absence of active BH3-only proteins.Cell Death Dis. 2015 Nov 26;6(11):e1996. doi: 10.1038/cddis.2015.341. Cell Death Dis. 2015. PMID: 26610208 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum.Autophagy. 2006 Jul-Sep;2(3):238-40. doi: 10.4161/auto.2730. Epub 2006 Jul 22. Autophagy. 2006. PMID: 16874066 Review.
References
-
- Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol.15, 49–63 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

