Patient Engagement and Patient Experience Data in Regulatory Review and Health Technology Assessment: Where Are We Today?
- PMID: 40210822
- PMCID: PMC12181203
- DOI: 10.1007/s43441-025-00770-6
Patient Engagement and Patient Experience Data in Regulatory Review and Health Technology Assessment: Where Are We Today?
Abstract
Background: As healthcare stakeholders aim to support patient-centered care, patients play an increasingly important role in pharmaceutical and medical technology development and healthcare decision-making. Patient engagement (PE), patient experience data (PED), and meaningful integration of PE to enrich PED have been evolving rapidly. This landscape review focuses on emerging PE/PED practices and guidelines in 2023.
Methods: References published between January-December 2023 on the use of PE and PED from health technology assessment (HTA) and regulatory bodies in different countries, three peer-reviewed journals, and referred resources from collaborators were analyzed. These references were compared with those in our previous publication (August 2021-January 2023, 17-month period).
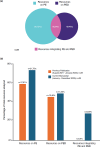
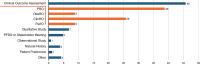
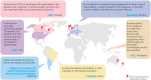
Results: Overall, 28 references from HTA/regulatory bodies, 26 from peer-reviewed articles, and 17 referred resources were identified. Eight references on PE and PED integration (PE + PED) were identified in 2023 from HTA/regulatory bodies, compared with none in the previous 17-month analysis. Emerging trends on the role of PE, PED, and real-world evidence in HTA/regulatory deliberations, transparency and geographic variations in the use of such evidence and practices, and gaps thereof have been highlighted.
Conclusions: The increase in PE, PED, and PE + PED references worldwide in 2023 versus the prior 17-month analysis suggests accelerated adoption of PE + PED practices. However, a need remains for comprehensive, actionable guidance on best practices for use of PE and PED for harmonization and incorporation into HTA/regulatory processes. Patient input-essential for evidence-based decision-making-provides valuable insights that enhance care quality, treatment relevance and effectiveness, and builds trust and sustainability.
Keywords: Health technology assessment; Patient engagement; Patient experience data; Real-world evidence; Regulatory assessment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: BL is an employee at Boehringer Ingelheim International GmbH. TW is an employee and shareholder of F. Hoffman-La Roche. No conflicts of interest are declared for NBertelsen, EO, TW-R, JE, NS, IS, OP, MMM, HC, or NBrooke.
Figures
References
-
- APACMed. Value-based pricing for medtech: A collaborative way forward for Singapore. (2023). https://apacmed.org/wp-content/uploads/2023/10/Value-Based_Pricing_in_Me.... Accessed January 28, 2025.
-
- U.S. Food, Drug Administration (FDA). December &. Draft guidance: Use of real-world evidence to support regulatory decision-making for medical devices. 2023. https://www.fda.gov/media/174819/download. Accessed January 28, 2025.
-
- Pushparajah D, Geissler J, Westergaard N, EUPATI. Collaborating between patients, academia and industry to champion the informed patient in medicines research and development. J Med Develop Sci. 2015;1. 10.18063/JMDS.2015.01.011.
-
- Canada’s Drug Agency (CDA). CDER patient-focused drug development. 2023. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patien.... Accessed January 28, 2025.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

