Allosteric targeted drug delivery for enhanced blood-brain barrier penetration via mimicking transmembrane domain interactions
- PMID: 40210849
- PMCID: PMC11986143
- DOI: 10.1038/s41467-025-58746-x
Allosteric targeted drug delivery for enhanced blood-brain barrier penetration via mimicking transmembrane domain interactions
Abstract
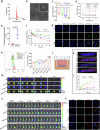
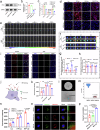
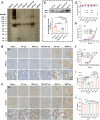
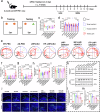
Current strategies for active targeting in the brain are entirely based on the effective interaction of the ligand with the orthosteric sites of specific receptors on the blood-brain barrier (BBB), which is highly susceptible to various pathophysiological factors and limits the efficacy of drug delivery. Here, we propose an allosteric targeted drug delivery strategy that targets classical BBB transmembrane receptors by designing peptide ligands that specifically bind to their transmembrane domains. This strategy prevents competitive interference from endogenous ligands and antibodies by using the insulin receptor and integrin αv as model targets, respectively, and can effectively overcome pseudotargets or target loss caused by shedding or mutating the extracellular domain of target receptors. Moreover, these ligands can be spontaneously embedded in the phospholipid layer of lipid carriers using a plug-and-play approach without chemical modification, with excellent tunability and immunocompatibility. Overall, this allosteric targeted drug delivery strategy can be applied to multiple receptor targets and drug carriers and offers promising therapeutic benefits in brain diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Targeting receptor-ligand chemistry for drug delivery across blood-brain barrier in brain diseases.Life Sci. 2021 Jun 1;274:119326. doi: 10.1016/j.lfs.2021.119326. Epub 2021 Mar 9. Life Sci. 2021. PMID: 33711385 Review.
-
Cellular and Molecular Targeted Drug Delivery in Central Nervous System Cancers: Advances in Targeting Strategies.Curr Top Med Chem. 2020;20(30):2762-2776. doi: 10.2174/1568026620666200826122402. Curr Top Med Chem. 2020. PMID: 32851962 Review.
-
Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier.Int J Pharm. 2019 Mar 25;559:360-372. doi: 10.1016/j.ijpharm.2019.01.056. Epub 2019 Feb 2. Int J Pharm. 2019. PMID: 30721725 Review.
-
Recent progress of drug nanoformulations targeting to brain.J Control Release. 2018 Dec 10;291:37-64. doi: 10.1016/j.jconrel.2018.10.004. Epub 2018 Oct 9. J Control Release. 2018. PMID: 30308256 Review.
-
Nanotechnologies: a strategy to overcome blood-brain barrier.Curr Drug Metab. 2012 Jan;13(1):61-9. doi: 10.2174/138920012798356943. Curr Drug Metab. 2012. PMID: 22292810 Review.
Cited by
-
Breaking barriers: exploring blood-brain barrier crossing mechanisms with nanomedicine for effective glioma treatment.3 Biotech. 2025 Jul;15(7):213. doi: 10.1007/s13205-025-04378-3. Epub 2025 Jun 13. 3 Biotech. 2025. PMID: 40521219 Review.
-
Mechanical Agitation-Assisted Transmembrane Drug Delivery by Magnetically Powered Spiky Nanorobots.Research (Wash D C). 2025 Aug 13;8:0768. doi: 10.34133/research.0768. eCollection 2025. Research (Wash D C). 2025. PMID: 40809455 Free PMC article.
References
-
- Ulbrich, K., Knobloch, T. & Kreuter, J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J. Drug Target.19, 125–132 (2011). - PubMed
-
- Montagut, C. et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat. Med.18, 221–223 (2012). - PubMed
-
- Wang, X. et al. Liposomes with cyclic RGD peptide motif triggers acute immune response in mice. J. Control. Release293, 201–214 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

