Novel derivatives of brincidofovir and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine inhibit orthopoxviruses and human adenoviruses more potently than brincidofovir
- PMID: 40210872
- PMCID: PMC11985979
- DOI: 10.1038/s41392-025-02207-w
Novel derivatives of brincidofovir and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine inhibit orthopoxviruses and human adenoviruses more potently than brincidofovir
Abstract
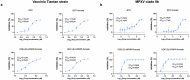
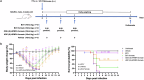
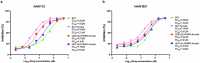
Brincidofovir (BCV) and tecovirimat are the only two chemical drugs that have been approved to treat smallpox and can be requested for monkeypox (Mpox) treatment through a single-patient Emergency Investigational New Drug (EIND) application. Disappointedly, the efficacy of tecovirimat manifested in recent clinical trials is far from being satisfactory, while the clinical efficacy of BCV is still inconclusive. Given that monkeypox virus (MPXV), variola and other emerging orthopoxviruses are posing serious threats to global health, it is urgent to develop better therapeutics. In this study, we tested the antiviral effects of three novel prodrugs, which were designed based on previously reported parent drugs, either (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine ((S)-HPMPC, cidofovir) or (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine ((S)-HPMPA). We found that one of the (S)-HPMPA-based prodrugs, ODE-(S)-HPMPA formate, exhibited significantly better anti-orthopoxvirus activity than BCV both in vitro and in vivo, which also inhibited human adenovirus type 2 and type 21 more efficiently than BCV. Most strikingly, the EC50 and EC90 of ODE-(S)-HPMPA formate against MPXV were more than 40-fold lower than those of BCV. In contrast, we observed that the anti-herpes simplex virus type 1 (HSV-1) activities of the (S)-HPMPA-based prodrugs were less effective than those of the cidofovir-based prodrugs (BCV and BCV formate), especially in vivo. Moreover, we showed for the first time that cytidine and adenine analog combined therapies could provide mice with complete protection against lethal challenges of both vaccinia and HSV-1. Collectively, we propose that both the ODE-(S)-HPMPA formate and the BCV/ODE-(S)-HPMPA formate combination are worth further investigations for their potential clinical applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Patent applications regarding the novel prodrugs evaluated in this study have been jointly filed by Shanghai Sci-Tech Inno Center for Infection & Immunity and Risen Pharma Tech Co., Ltd.. The authors declare no other competing interests.
Figures



Similar articles
-
Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model.mSphere. 2021 Feb 3;6(1):e00927-20. doi: 10.1128/mSphere.00927-20. mSphere. 2021. PMID: 33536322 Free PMC article.
-
Tyrosine-based 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine and -adenine ((S)-HPMPC and (S)-HPMPA) prodrugs: synthesis, stability, antiviral activity, and in vivo transport studies.J Med Chem. 2011 Aug 25;54(16):5680-93. doi: 10.1021/jm2001426. Epub 2011 Aug 3. J Med Chem. 2011. PMID: 21812420 Free PMC article.
-
Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro.J Infect Dis. 2005 Feb 1;191(3):396-9. doi: 10.1086/426831. Epub 2004 Dec 29. J Infect Dis. 2005. PMID: 15633099
-
In vitro activity of potential anti-poxvirus agents.Antiviral Res. 2003 Jan;57(1-2):35-40. doi: 10.1016/s0166-3542(02)00198-5. Antiviral Res. 2003. PMID: 12615301 Free PMC article. Review.
-
Progress in the discovery of compounds inhibiting orthopoxviruses in animal models.Antivir Chem Chemother. 2008;19(3):115-24. doi: 10.1177/095632020801900302. Antivir Chem Chemother. 2008. PMID: 19024628 Review.
References
-
- Petersen, B. W., Karem, K. L. & Damon, I. K. in Viral Infections of Humans, (eds Kaslow, R., Stanberry, L., & Le Duc, J.) 501-517 (Springer, 2014).
-
- Berche, P. The threat of smallpox and bioterrorism. Trends Microbiol.9, 15–18 (2001). - PubMed
-
- Tegnell, A., Wahren, B. & Elgh, F. Smallpox–eradicated, but a growing terror threat. Clin. Microbiol. Infect.8, 504–509 (2002). - PubMed
-
- Lu, L., Su, S., Yang, H. & Jiang, S. Antivirals with common targets against highly pathogenic viruses. Cell184, 1604–1620 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

