Involvement of circadian clock protein PER2 in controlling sleep deprivation induced HMGB1 up-regulation by targeting p300 in the cortex
- PMID: 40210902
- PMCID: PMC11985928
- DOI: 10.1038/s41598-025-96931-6
Involvement of circadian clock protein PER2 in controlling sleep deprivation induced HMGB1 up-regulation by targeting p300 in the cortex
Abstract
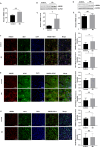
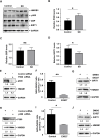
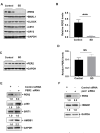
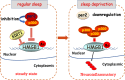
Lack of sleep is a common problem in current society, which can induce various brain dysfunctions. Neuroinflammation is a typical reaction caused by sleep deficit and is considered as a common basis for various neurological disorders and cognitive impairments, but the related mechanisms have not been fully clarified. The circadian clock protein plays a critical role in maintaining physiological homeostasis, including sleep/wake cycles. Circadian disorders induced by sleep deficit might contribute to the development of neuroinflammation. In the current study, we observed that sleep deprivation (SD) induced elevated expression of High-mobility group box 1 (HMGB1), one of the most important mediators of neuroinflammation, in the cortical microglia and cerebrospinal fluids. Moreover, acetylation-dependent nuclear export of HMGB1 was involved in up-regulation and secretion of HMGB1 after sleep deprivation. Further studies indicated that sleep deprivation induced an increase in the expression of acetyltransferase p300 and a decrease in the expression of deacetylase SIRT1, which synergistically enhanced the acetylation level of HMGB1 in the cortical microglial cells, thereby triggered the nuclear export and secretion of HMGB1. Most importantly, circadian clock protein PER2 constitutively interacted with p300 and inhibited its expression in the microglial cells, which can be interrupted by PER2 downregulation upon sleep deprivation, leading to the increased expression of p300 and acetylation and secretion of HMGB1. The truncated PER2 mutant without p300 binding ability lost its ability to regulate p300 expression, indicating that PER2 functioned as a co-suppressor of p300 in regulating acetylation and expression of HMGB1. Taken together, data in this study reveal a new mechanism by which PER2 is involved in controlling HMGB1 dependent neuroinflammation induced by sleep deprivation. Maintaining PER2 levels or blocking HMGB1 acetylation in the cortex might be prospective for preventing sleep deprivation-induced neuroinflammation and the related adverse reactions in the brain.
Keywords: HMGB1; Neuroinflammation; PER2; Sleep deprivation; p300.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This study was performed in line with the international guidelines for the care and use of laboratory animals. Approval was granted by the by the Animal Ethics Committee of Beijing Institute of Basic Medical Sciences (No. IACUC-DWZX-2023-546).
Figures


References
-
- Perlis, M. L. et al. Insomnia Lancet400, 1047–1060 10.1016/s0140-6736(22)00879-0 (2022). - PubMed
-
- Faraut, B., Boudjeltia, K. Z., Vanhamme, L. & Kerkhofs, M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep. Med. Rev.16, 137–149. 10.1016/j.smrv.2011.05.001 (2012). - PubMed
-
- Liew, S. C. & Aung, T. Sleep deprivation and its association with diseases- a review. Sleep. Med.77, 192–204. 10.1016/j.sleep.2020.07.048 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

