The human brainstem's red nucleus was upgraded to support goal-directed action
- PMID: 40210909
- PMCID: PMC11986128
- DOI: 10.1038/s41467-025-58172-z
The human brainstem's red nucleus was upgraded to support goal-directed action
Abstract
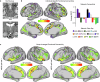
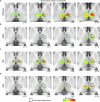
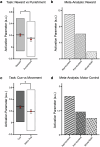
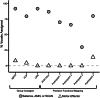
The red nucleus, a large brainstem structure, coordinates limb movement for locomotion in quadrupedal animals. In humans, its pattern of anatomical connectivity differs from that of quadrupeds, suggesting a different purpose. Here, we apply our most advanced resting-state functional connectivity based precision functional mapping in highly sampled individuals (n = 5), resting-state functional connectivity in large group-averaged datasets (combined n ~ 45,000), and task based analysis of reward, motor, and action related contrasts from group-averaged datasets (n > 1000) and meta-analyses (n > 14,000 studies) to precisely examine red nucleus function. Notably, red nucleus functional connectivity with motor-effector networks (somatomotor hand, foot, and mouth) is minimal. Instead, connectivity is strongest to the action-mode and salience networks, which are important for action/cognitive control and reward/motivated behavior. Consistent with this, the red nucleus responds to motor planning more than to actual movement, while also responding to rewards. Our results suggest the human red nucleus implements goal-directed behavior by integrating behavioral valence and action plans instead of serving a pure motor-effector function.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Within the last year, J.S.Siegel was an employee of Sumitomo Pharma America and received consulting fees from Otsuka and Longitude Capital. D.A.F., and N.U.F.D. have a financial interest in Turing Medical Inc. and may benefit financially if the company is successful in marketing FIRMM motion monitoring software products. J.T.W. is a consultant for Turing Medical Inc. A.N.V., E.M.G., D.A.F. and N.U.F.D. may receive royalty income based on technology developed at Washington University School of Medicine and Oregon Health and Sciences University and licensed to Turing Medical Inc. D.A.F. and N.U.F.D. are co-founders of Turing Medical Inc. These potential conflicts of interest have been reviewed and are managed by Washington University School of Medicine, Oregon Health and Sciences University and the University of Minnesota. The other authors declare no competing interests.
Figures
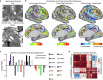
Update of
-
The brainstem's red nucleus was evolutionarily upgraded to support goal-directed action.bioRxiv [Preprint]. 2024 Jan 1:2023.12.30.573730. doi: 10.1101/2023.12.30.573730. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 10;16(1):3398. doi: 10.1038/s41467-025-58172-z. PMID: 38260662 Free PMC article. Updated. Preprint.
Similar articles
-
The brainstem's red nucleus was evolutionarily upgraded to support goal-directed action.bioRxiv [Preprint]. 2024 Jan 1:2023.12.30.573730. doi: 10.1101/2023.12.30.573730. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 10;16(1):3398. doi: 10.1038/s41467-025-58172-z. PMID: 38260662 Free PMC article. Updated. Preprint.
-
[Study of resting state functional connectivity of the red nucleus and substantia nigra were in normal adult].Zhonghua Yi Xue Za Zhi. 2013 Dec 17;93(47):3758-61. Zhonghua Yi Xue Za Zhi. 2013. PMID: 24548392 Chinese.
-
Is Rest Really Rest? Resting-State Functional Connectivity During Rest and Motor Task Paradigms.Brain Connect. 2018 Jun;8(5):268-275. doi: 10.1089/brain.2017.0495. Brain Connect. 2018. PMID: 29665711
-
Love is analogous to money in human brain: Coordinate-based and functional connectivity meta-analyses of social and monetary reward anticipation.Neurosci Biobehav Rev. 2019 May;100:108-128. doi: 10.1016/j.neubiorev.2019.02.017. Epub 2019 Feb 23. Neurosci Biobehav Rev. 2019. PMID: 30807783 Free PMC article. Review.
-
In vivo structural and functional imaging of the human rubral and inferior olivary nuclei: A mini-review.Cerebellum. 2010 Jun;9(2):167-73. doi: 10.1007/s12311-009-0145-1. Cerebellum. 2010. PMID: 19898914 Review.
References
-
- Baizer, J. Unique Features of the Human Brainstem and Cerebellum. Front. Hum. Neurosci. 8https://www.frontiersin.org/journals/humanneuroscience/articles/10.3389/... (2014). - DOI - PMC - PubMed
-
- Baizer, J. S., Webster, C. J. & Witelson, S. F. Individual variability in the size and organization of the human arcuate nucleus of the medulla. Brain Struct. Funct.227, 159–176 (2022). - PubMed
-
- Sara, S. J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci.10, 211–223 (2009). - PubMed
-
- De Lange, S. J. The red nucleus in reptiles. K. Ned. Akad. Van. Wet. Proc. Ser. B Phys. Sci.14, 1082–1090 (1912).
MeSH terms
Grants and funding
- R01 MH118388/MH/NIMH NIH HHS/United States
- R01 DA047851/DA/NIDA NIH HHS/United States
- K23 NS123345/NS/NINDS NIH HHS/United States
- K23 NS133486/NS/NINDS NIH HHS/United States
- R01 MH096773/MH/NIMH NIH HHS/United States
- R00 MH129616/MH/NIMH NIH HHS/United States
- T32DA007261/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- T32 DA007261/DA/NIDA NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- R56 MH114976/MH/NIMH NIH HHS/United States
- R44 NS129521/NS/NINDS NIH HHS/United States
- R01 NS140256/NS/NINDS NIH HHS/United States
- R01 NS124789/NS/NINDS NIH HHS/United States
- K99 MH129616/MH/NIMH NIH HHS/United States
- F32 MH120989/MH/NIMH NIH HHS/United States
- R01 MH120194/MH/NIMH NIH HHS/United States
- K12 NS098482/NS/NINDS NIH HHS/United States
- R44 MH121276/MH/NIMH NIH HHS/United States
- R01 MH115357/MH/NIMH NIH HHS/United States
- R44 MH124567/MH/NIMH NIH HHS/United States
- R44 MH122066/MH/NIMH NIH HHS/United States

