CircSCD1 inhibits ferroptosis in breast Cancer through stabilizing SCD1 protein via deubiquitinase OTUB1
- PMID: 40210941
- PMCID: PMC11985923
- DOI: 10.1038/s41598-025-96868-w
CircSCD1 inhibits ferroptosis in breast Cancer through stabilizing SCD1 protein via deubiquitinase OTUB1
Abstract
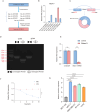
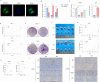
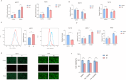
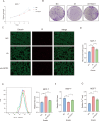
Breast cancer is the leading cause of cancer-related death in women worldwide, and its developmental mechanisms involve complex factors. Recent studies have shown that ferroptosis is closely related to the occurrence and progression of breast cancer. However, the role of circular RNAs (circRNAs) in regulating ferroptosis in breast cancer remains unclear. In this study, we investigated the regulatory role of circSCD1 (hsa_circ_0019512) in breast cancer. We examined the expression of circSCD1 in breast cancer cell lines and explored its impact on cell viability and colony formation. We also evaluated the involvement of circSCD1 in ferroptosis by measuring the levels of malondialdehyde (MDA), glutathione (GSH), reactive oxygen species (ROS), and intracellular iron. In vivo xenograft experiments were performed to confirm the role of circSCD1 in promoting tumor growth and inhibiting ferroptosis.Furthermore, we investigated the mechanism by which circSCD1 regulates SCD1 protein stability through ubiquitination and identified the interaction between circSCD1 and the deubiquitinase OTUB1. Our results showed that circSCD1 was upregulated in breast cancer cell lines and promoted cell viability and colony formation. Knockdown of circSCD1 increased MDA and ROS levels, decreased GSH levels, and enhanced ferroptosis in breast cancer cells. In vivo, circSCD1 knockdown significantly reduced tumor size and weight, while its overexpression enhanced tumor growth. Mechanistically, circSCD1 interacted with OTUB1 to inhibit the ubiquitination and degradation of SCD1 protein, thereby stabilizing its expression. Rescue experiments demonstrated that SCD1 overexpression partially reversed the effects of circSCD1 knockdown on cell proliferation and ferroptosis. Our findings suggest that circSCD1 plays a crucial role in promoting breast cancer cell growth and inhibiting ferroptosis by regulating SCD1 protein stability. Targeting the circSCD1/OTUB1/SCD1 axis may provide a potential therapeutic strategy for breast cancer treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests. Ethical approval: All animal experiments were conducted in compliance with the ARRIVE guidelines ( https://arriveguidelines.org ). The experimental protocols were approved by the Medical Ethics Committee of Xiangya Hospital, Central South University (Approval No. XY20240521005). Every effort was made to minimize animal suffering during the study, including the implementation of humane endpoints and appropriate anesthesia protocols.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

