D-chiro-inositol effectively counteracts endometriosis in a mouse model
- PMID: 40211112
- PMCID: PMC11987403
- DOI: 10.1186/s10020-025-01178-6
D-chiro-inositol effectively counteracts endometriosis in a mouse model
Abstract
Background: Endometriosis, a common condition affecting 5-10% of women of reproductive age, is the growth of endometrial-like tissue outside the uterus, leading to pain and infertility. Current treatments, such as surgery and hormonal therapy, offer limited long-term benefits. This study investigated the potential of D-chiro inositol (DCI), a natural compound that influences ovarian steroidogenesis, to treat endometriosis and compared its efficacy with a progestin drug such as Dienogest (DG).
Methods: We established a non-surgical mouse model of endometriosis in CD1 mice. Uterine horns were removed from donor mice, cut into fragments and inoculated in recipient mice by intraperitoneal injection. Endometriosis progression was assessed at 15, 21 and 28 days after transplantation, with the 28-day window being the most effective. The mice were then randomly assigned to four experimental groups, which received for 28 days: water (EMS); DCI 0.4 mg/die (DCI); DCI 0.2 mg/die and Dienogest 0.33 ng/die (DCI + DG); DG 0.67 ng/die (DG). At the end of the treatments, endometriotic lesions, ovaries and circulating estradiol levels were analyzed.
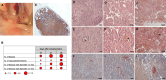
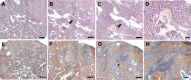
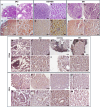
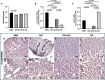
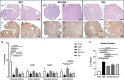
Results: The results showed that treatment with DCI, both alone and in combination with DG, significantly reduced the number, size and vascularization of endometriotic lesions compared to the EMS control group. Histological analysis confirmed a decrease in endometriotic foci across all treatment groups, with the most pronounced effects in the DCI group. To investigate the underlying molecular mechanisms, we found that DCI led to a significant reduction in the expression of Sirt1 and an increase in E-Cadherin, indicating a reduction in EMT transition relevant for lesion development. In addition, DCI decreased cell proliferation and,blood vessel formation, as evaluated by PCNA and CD34, respectively. Futhermore, in the ovary, DCI treatment downregulated the expression of aromatase (Cyp19a1), the enzyme critical for estrogen biosynthesis, and increased the number of primordial to antral follicles, suggesting a beneficial effect on ovarian folliculogenesis.
Conclusions: By modulating proliferation, EMT transition and aromatase activity, DCI emerges as a promising compound for endometriosis treatment.
Keywords: Aromatase; DCI; DG; E-Cadherin; EMT; Endometriosis; Sirt1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The project was approved by the Italian Ministry of Health and the internal Committee of the University of L’Aquila (authorization no. 917/2023-PR). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Ahmed I, Balestrieri E, Tudosa I, Lamonaca F. Morphometric measurements of blood cell. Meas Sens. 2021;18:100294. 10.1016/j.measen.2021.100294.
-
- Amaral C, Augusto TV, Almada M, et al. The potential clinical benefit of targeting androgen receptor (AR) in estrogen-receptor positive breast cancer cells treated with Exemestane. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165661. 10.1016/j.bbadis.2019.165661. - PubMed
-
- Ansieau S, Bastid J, Doreau A, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. 10.1016/j.ccr.2008.06.005. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

