Effect of hydromorphone combined with ropivacaine caudal block on immune function after hypospadias surgery in children
- PMID: 40211132
- PMCID: PMC11987407
- DOI: 10.1186/s12871-025-03053-7
Effect of hydromorphone combined with ropivacaine caudal block on immune function after hypospadias surgery in children
Abstract
Background: This study aimed to evaluate the effects of caudal block anesthesia with hydromorphone-ropivacaine compared to ropivacaine alone on postoperative immune function and pain management in children undergoing hypospadias surgery.
Methods: A total of 100 pediatric patients were randomly assigned to two groups: the Hydromorphone-Ropivacaine (HR) group and the Ropivacaine (R) group for caudal block anesthesia, with 50 patients in each group. The R group received 0.25% ropivacaine at a dose of 1 ml/kg, while the HR group received 0.25% ropivacaine (1 ml/kg) combined with hydromorphone (10 µg/kg). The maximum dose for both groups was capped at 30 ml (1 ml/kg). Anesthesia induction included intravenous administration of pentobarbital (0.01 mg/kg) and dexamethasone (0.15 mg/kg), followed by sevoflurane inhalation. All patients underwent ultrasound-guided caudal block anesthesia administered by the same anesthetist. Primary outcomes included the distribution of T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8 + ratios) measured at five time points: pre-anesthesia (T0), end of surgery (T1), 24 h postoperatively (T2), 48 h postoperatively (T3), and 72 h postoperatively (T4). Secondary outcomes included postoperative pain scores assessed using the Modified Children's Hospital of Eastern Ontario Pain Scale (M-CHEOPS) at 1, 6, 12, 18, and 24 h postoperatively, sedation levels evaluated using the Ramsay sedation scale at the same time points, and the incidence of postoperative adverse events.
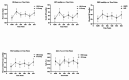
Results: The HR group exhibited significant reductions in CD3+, CD4+, and CD4+/CD8+ ratios at T1, T2, and T3 compared to baseline (T0) (p < 0.001). At all postoperative time points (T1, T2, T3, T4), the HR group demonstrated significantly higher levels of CD3+, CD4+, and CD4+/CD8+ ratios compared to the R group (p < 0.001). By T4 (72 h postoperatively), immune markers in the HR group had largely normalized to baseline levels, whereas those in the R group remained significantly lower (p < 0.001). Postoperative pain, assessed using the Modified Children's Hospital of Eastern Ontario Pain Scale (M-CHEOPS), was significantly lower in the HR group at 6, 12, and 18 h postoperatively compared to the R group (p < 0.001). The HR group also exhibited a longer duration of analgesia and required fewer doses of rescue analgesia within the first 24 h postoperatively (p = 0.046). Sedation levels, evaluated using the Ramsay sedation scale, showed significant differences between the groups at 1 h (p = 0.0087) and 6 h (p < 0.0001) postoperatively, with higher sedation scores observed in the HR group. There were no significant differences in heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure, or oxygen saturation between the groups at any time point (p > 0.05). No significant differences were observed between the two groups in terms of postoperative adverse reactions (all p > 0.05).
Conclusion: Caudal block anesthesia with hydromorphone-ropivacaine offers enhanced postoperative pain relief and a lesser impact on immune function compared to ropivacaine alone in children undergoing hypospadias surgery. Further studies are warranted to explore the long-term effects on immune function.
Keywords: Anesthesia; Caudal block; Hydromorphone; Hypospadias surgery; Immune function; Ropivacaine; T lymphocyte subsets.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Anhui Children’s Hospital Ethics Committee (Ethics Number: ETYY-2022-025). Informed consent was obtained from all participants or their legal guardians prior to participation in the study. The purpose, procedures, potential risks, and benefits of the study were explained to each participant, and written informed consent was obtained. For participants under the age of 18, informed consent was obtained from their parents or legal guardians. All personal data were handled confidentially, and all efforts were made to ensure the privacy and anonymity of the participants throughout the study. It was registered with the Chinese Clinical Trial Registry ( https://www.chictr.org.cn ) with registration number: ChiCTR2300072599, registration date: 2023-06-19. Consent for publication: This study does not contain any individual person’s data in any form (including any individual details, images or videos) that compromise anonymity. Therefore, it is not applicable to include a consent for publication statement in this section. Competing interests: The authors declare no competing interests. Clinical implications: a. What is already known about the topic: Caudal block anesthesia, particularly when combined with general anesthesia, has been shown to effectively reduce postoperative pain and limit immunosuppression in pediatric surgeries by lowering the release of stress hormones and inflammatory mediators. b. What new information this study adds: This study demonstrates that the combination of hydromorphone with ropivacaine for caudal block anesthesia results in superior postoperative pain relief and a faster recovery of immune function, as indicated by higher levels of T lymphocyte subsets (CD3+, CD4+, and CD4+/CD8+ ratios) compared to ropivacaine alone. In accordance with BMC Series editorial policies, this study adheres to the CONSORT guidelines for reporting clinical trials. A completed CONSORT checklist is available as a supplementary file.
Figures
References
-
- Rasool N. Outcome of hypospadias surgery in children. TPMJ. 2024;26:3368.
-
- Soave A, Riechardt S, Engel O, et al. Komplikationen Bei Hypospadiekorrekturen Urologe A. 2014;53(7):1001–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

