Resveratrol attenuates the CoCl2-induced hypoxia damage by regulation of lysine β-hydroxybutyrylation in PC12 cells
- PMID: 40211144
- PMCID: PMC11984057
- DOI: 10.1186/s12883-025-04171-y
Resveratrol attenuates the CoCl2-induced hypoxia damage by regulation of lysine β-hydroxybutyrylation in PC12 cells
Abstract
Background: Stroke is a cerebrovascular disease that is the main cause of death and disability worldwide. Hypoxia is a major factor that causes neuronal damage and even cellular death. However, the mechanism and therapeutic drugs for hypoxia are not completely understood.
Methods: In this study, PC12 cells (a rat adrenal pheochromocytoma cell line) were exposed to Cobalt chloride (CoCl2) to induce hypoxia. Using this cell model, the impacts of hypoxia on cell viability, proliferation, reactive oxygen species (ROS), and the levels of lysine β-hydroxybutyrylation (Kbhb) and the inflammatory signaling factor P65 were examined. In addition, we explored the ability of resveratrol (RES) to alleviate CoCl2-induced hypoxia damage.
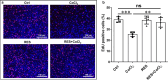
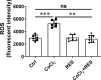
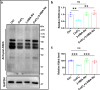
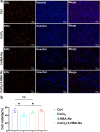
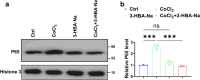
Results: RES attenuated CoCl2-induced decreases of cell viability and cell proliferation and increase of ROS production in PC12 cells. CoCl2 downregulated Kbhb in PC12 cells, but RES alleviated this effect. In addition, upregulated Kbhb by 3-hydroxybutyric acid sodium could partially recover the CoCl2-induced hypoxia damage to PC12 cells, including cell viability, cell proliferation, oxidative stress, and the protein level of the inflammatory signaling factor P65.
Conclusion: Our results indicate that RES protects against CoCl2-induced hypoxia damage in PC12 cells by modulating Kbhb, a novel post-translational modification.
Keywords: CoCl2; Hypoxia; Lysine β-hydroxybutyrylation; PC12 cells; Reactive oxygen species; Resveratrol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

