Integrated m6A RNA methylation and transcriptomic analysis of Apostichopus japonicus under combined high-temperature and hypoxia stress
- PMID: 40211154
- PMCID: PMC11987279
- DOI: 10.1186/s12864-025-11532-x
Integrated m6A RNA methylation and transcriptomic analysis of Apostichopus japonicus under combined high-temperature and hypoxia stress
Abstract
Background: Global climate change has significantly increased environmental stress in marine ecosystems, with rising sea surface temperatures and declining dissolved oxygen (DO) levels. These stressors pose critical challenges to aquaculture, particularly for Apostichopus japonicus, an economically significant species in China. A. japonicus is highly sensitive to combined high-temperature and hypoxia stress, which disrupts physiological processes, suppresses immune responses, and increases mortality. While epigenetic mechanisms such as N6-methyladenosine (m6A) RNA modifications are known to regulate stress adaptation, their role under dual stressors in A. japonicus remains poorly understood.
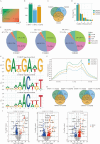
Results: This study integrates m6A methylation sequencing (MeRIP-seq) and transcriptomic analysis (RNA-seq) to investigate molecular responses in A. japonicus under combined high-temperature (32 °C) and hypoxia (DO = 2 mg/L). Results show that approximately 90% of genes had 1-3 m6A peaks, with single peaks being the most frequent (∼ 60%). Genes with m6A modifications exhibited varying expression levels, with some showing significantly higher expression, suggesting a complex relationship between m6A methylation and stress-responsive gene expression. GO and KEGG enrichment analyses revealed that m6A-modified genes regulate pathways associated with oxidative stress, protein homeostasis, and energy metabolism, such as the PI3K-Akt and MAPK signaling pathways. Key stress-responsive genes, including HSP70, NOX5, and SLC7A11, exhibited dynamic m6A methylation changes, highlighting their roles in redox homeostasis and cellular resilience. Comparative analysis across experimental groups revealed distinct molecular responses to hypoxia, high-temperature stress, and their combination, with combined stress inducing more pronounced changes in m6A methylation and gene expression.
Conclusion: In this study, we explored the central regulatory role of m6A RNA methylation in the response of A. japonicus to the dual environmental stress of high-temperature and hypoxia. The findings show that m6A modification regulates the expression of key genes, allowing A. japonicus to effectively adapt to harsh environmental conditions. This study not only provides an important new perspective on the molecular stress recovery mechanism of marine invertebrates in the face of complex environmental stress, but it also provides theoretical support for aquaculture practice, assisting in the development of more stress-resistant aquaculture systems to deal with the severe challenges posed by global climate change.
Keywords: Apostichopus japonicus; High-temperature stress; Hypoxia stress; Stress adaptation mechanisms; m6A RNA methylation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal study was approved by the Institutional Animal Care and Use Committee of the Ludong University (protocol number LDU-IRB20210308NXY). The study was conducted in accordance with the local legislation and institutional requirements. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Li Y, et al. High-quality sea surface temperature measurements along Coast of the Bohai and yellow seas in China and their long‐term trends during 1960–2012. Int J Climatol. 2019;40:63–76. - DOI
-
- Shi W, Wang M. Satellite views of the Bohai Sea, yellow Sea, and East China Sea - ScienceDirect. Prog Oceanogr. 2012;104(10):30–45. - DOI
-
- Chen Y, et al. Seasonal variability in dissolved oxygen in the Bohai Sea, China. J Oceanol Limnol. 2022;40(1):78–92. - DOI
-
- Kang B, et al. Climate change impacts on China’s marine ecosystems. Rev Fish Biol Fish. 2021;31:599–629. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

