A Phase Ia/b study of MEK1/2 inhibitor binimetinib with MET inhibitor crizotinib in patients with RAS mutant advanced colorectal cancer (MErCuRIC)
- PMID: 40211189
- PMCID: PMC11984268
- DOI: 10.1186/s12885-025-14068-1
A Phase Ia/b study of MEK1/2 inhibitor binimetinib with MET inhibitor crizotinib in patients with RAS mutant advanced colorectal cancer (MErCuRIC)
Abstract
Background: Targeting RAS mutant (MT) colorectal cancer (CRC) remains a difficult challenge, mainly due to the pervasiveness of RAS/MEK-mediated feedback loops. Preclinical studies identified MET/STAT3 as an important mediator of resistance to KRAS-MEK1/2 blockade in RASMT CRC. This dose escalation/expansion study assessed safety and initial efficacy of the MEK1/2 inhibitor binimetinib with MET inhibitor crizotinib in RASMT advanced CRC patients.
Methods: In the dose escalation phase, patients with advanced solid tumours received binimetinib with crizotinib, using a rolling- 6 design to determine the maximum tolerable dose (MTD) and safety/tolerability. A subsequent dose expansion in RASMT CRC patients assessed treatment response. Blood samples for pharmacokinetics, MET biomarker and ctDNA analyses, and skin/tumour biopsies for pharmacodynamics, c-MET immunohistochemistry (IHC), MET in situ hybridisation (ISH) and MET DNA-ISH analyses were collected.
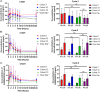
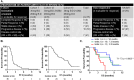
Results: Twenty patients were recruited in 3 cohorts in the dose escalation. The MTD was binimetinib 30 mg B.D, days 1-21 every 28 days, with crizotinib 250 mg O.D continuously. Dose-limiting toxicities included grade ≥ 3 transaminitis, creatinine phosphokinase increases and fatigue. Thirty-six RASMT metastatic CRC patients were enrolled in the dose expansion. Pharmacokinetic and pharmacodynamic parameters showed evidence of target engagement. Across the entire study, the most frequent treatment-related adverse events (TR-AE) were rash (80.4%), fatigue (53.4%) and diarrhoea (51.8%) with grade ≥ 3 TR-AE occurring in 44.6%. Best clinical response within the RASMT CRC cohort was stable disease in seven patients (24%). Tumour MET super-expression (IHC H-score > 180 and MET ISH + 3) was observed in 7 patients (24.1%), with MET-amplification only present in 1 of these patients. This patient discontinued treatment early during cycle 1 due to toxicity. Patients with high baseline RASMT allele frequency had a significant shorter median overall survival compared with that seen for patients with low baseline KRASMT allele frequency.
Conclusions: Combination binimetinib/crizotinib showed a poor tolerability with no objective responses observed in RASMT advanced CRC patients. EudraCT-Number: 2014-000463 - 40 (20/06/2014: A Sequential Phase I study of MEK1/2 inhibitors PD- 0325901 or Binimetinib combined with cMET inhibitor Crizotinib in RAS Mutant and RAS Wild Type with aberrant c-MET).
Keywords: Binimetinib; Colorectal cancer; Crizotinib; CtDNA; MET biomarker; Pharmacodynamics; Pharmacokinetics; Phase I; RAS mutant.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients provided written informed consent before participating in any study-related procedure. An ethics review committee approved the protocol (NRES Committee South Central—Oxford C REC reference number 14/SC/1010). Informed consent documentation was reviewed and approved by the institutional review board(s) or independent ethics committee(s) at each institution/country. The trial was authorised by the Medicines and Healthcare products Regulatory Agency in the UK and applicable competent authorities in each of the participating countries. The study was conducted as part of the portfolio of trials in the registered UKCRC Oxford Clinical Trials Research Unit at the University of Oxford. It followed their Standard Operating Procedures ensuring compliance with the principles of Good Clinical Practice and the Declaration of Helsinki and any applicable regulatory requirements. Consent for publication: All data has been anonymised and the manuscript does not contain any individually identifiable data. Not applicable. Competing interests: FA, EE, GP, HP, SVS, BTH, VP, PG, JH, LC, CR, CR, FDN, MG, RB, KB, JD, VC, ML, HK, MST, PLP, MRM, TSM, RA, RJ, MP, RHW: No competing interests connected with this study. MRM: supported by the NIHR Biomedical Research Centre at Oxford. The views expressed in this article are those of the authors, not necessarily those of the National Health Service, the NIHR, or the Department of Health. JT: scientific consultancy: Alentis-Therapeutics/AstraZeneca/Aveo-Oncology/Boehringer-Ingelheim/Cardiff-oncology/CARSgen-Therapeutics/Chugai/Daiichi-Sankyo/Hoffmann-La Roche-Ltd/Genentech-Inc/hC.Bioscience/Immodulon-Therapeutics/Inspirna-Inc/Lilly/Menarini/Merck-Serono/Merus/MSD/Mirati/Neophore/Novartis/Ona Therapeutics/Ono-Pharma-USA/Orion-Biotechnology/Peptomyc/Pfizer/Pierre-Fabre/Samsung-Bioepis/Sanofi/Scandion-Oncology/Scorpion-Therapeutics/Seattle-Genetics/Servier/Sotio-Biotech/Taiho/Takeda-Oncology/Tolremo-Therapeutics. Stocks: Oniria-Therapeutics/Alentis-Therapeutics/Pangaea-Oncology/1 TRIALSP. Educational collaboration: Medscape Education/PeerView Institute for Medical Education and Physicians Education Resource. TA: advisory board meetings/consulting fees: Abbvie/Aptitude Health/BMS/Gritstone-Oncology/Gilead/GlaxoSmithKline/Merck&Co.Inc./Nordic-Oncology/Pfizer/Seagen/Servier/Takeda. Honoraria: BMS/GlaxoSmithKline/Merck&Co. Inc./Merck-Serono/Roche/Sanofi/Seagen/Servier. DMC member role: Inspirna. Supported BMS/Merck&Co.Inc./Takeda meetings. A.B: grants from Neophore/AstraZeneca/Boehringer-Ingelheim. Honoraria/consultation fees: Guardant-Health. Stock shareholder: Neophore, Kither Biotech. Advisory board member: Neophore.
Figures

References
-
- Stintzing S, Miller-Phillips L, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50–60. - PubMed
-
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. - PubMed
-
- Kubicka S, Greil R, Andre T, Bennouna J, Sastre J, Van Cutsem E, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24:2342–9. - PubMed
-
- Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. - PubMed
-
- Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- 602901/FP7 Health
- C13749/A7261/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

