Vorinostat impairs the cancer-driving potential of leukemia-secreted extracellular vesicles
- PMID: 40211278
- PMCID: PMC11987450
- DOI: 10.1186/s12967-025-06361-1
Vorinostat impairs the cancer-driving potential of leukemia-secreted extracellular vesicles
Abstract
Background: Leukemia-secreted extracellular vesicles (EVs) carry biologically active cargo that promotes cancer-supportive mechanisms, including aberrant proliferative signaling, immune escape, and drug resistance. However, how antineoplastic drugs affect EV secretion and cargo sorting remains underexplored.
Methods: Leukemia-secreted extracellular vesicles (EVs) were isolated by Differential UltraCentrifugation, and their miRNome and proteomic profiling cargo were analyzed following treatment with SAHA (Vorinostat) in Acute Myeloid Leukemia (AML) and Chronic Myeloid Leukemia (CML). The epigenetic modulation of leukemia-secreted EVs content on interesting key target molecules was validated, and their differential functional impact on cellular viability, cell cycle progression, apoptosis, and tumorigenicity was assessed.
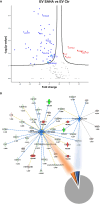
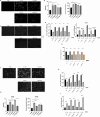
Results: SAHA significantly alters the cargo of Leukemia-derived EVs, including miR-194-5p and its target BCLAF1 (mRNA and protein), key regulators of Leukemia cell survival and differentiation. SAHA upregulates miR-194-5p expression while selective loading BCLAF1 into EVs, reducing the miRNA levels in the same compartment. Additionally, SAHA alters miRNA profile and proteomic composition associated with leukemic EVs, altering their tumor-supportive potential, with differential effects observed between AML and CML. Furthermore, in silico predictions suggest that these modified EVs may influence cell sensitivity to antineoplastic agents, suggesting a dual role for SAHA in impairing oncogenic signaling while enhancing therapeutic responsiveness.
Conclusions: In conclusion, the capacity of SAHA to modulate secretion and molecular composition of Leukemia-secreted EVs, alongside its direct cytotoxic effects, underscores its potential in combination therapies aimed to overcoming refractory phenotype by targeting EV-mediated communication.
Keywords: Epigenetics; Extracellular vesicles; Leukemia; SAHA; Tumorigenicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have read and agreed to the published version of the manuscript. Competing interests: The authors declare no competing interests.
Figures


References
-
- Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–36. - PubMed
-
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. - PubMed
MeSH terms
Substances
Grants and funding
- FISR2019_00374 MeDyCa-B84G19000200008/Ministero dell'Istruzione, dell'Università e della Ricerca
- Prin 2022 PNRR-SOLAR-B53D23025100001/Ministero dell'Istruzione, dell'Università e della Ricerca
- Nabucco no. 1682/Ministero delle Imprese e del Made in Italy
- EPIGENIUS-SARS-CoV-2-E93C22001650002/POR-FESR
- Law Decree 6 May 2021/The National Recovery and Resilience Plan (NRRP)
LinkOut - more resources
Full Text Sources
Medical

