Metabolomic signatures in liquid biopsy are associated with overall survival in metastatic melanoma patients treated with immune checkpoint inhibitor therapy
- PMID: 40211360
- PMCID: PMC11983745
- DOI: 10.1186/s13046-025-03378-8
Metabolomic signatures in liquid biopsy are associated with overall survival in metastatic melanoma patients treated with immune checkpoint inhibitor therapy
Abstract
Background: Immune checkpoint inhibitors (ICIs), such as anti-Cytotoxic T-Lymphocyte Antigen 4(CTLA-4) and anti-Programmed cell death protein 1 (PD-1) agents, have improved the prognosis of patients with metastatic melanoma. However, a proportion of patients develop resistance to these treatments, leading to a poor prognosis. Therefore, identifying potential non invasive and easy to measure biomarkers is crucial for guiding treatment strategies in patients with metastatic melanoma.
Methods: A retrospective single-center study was conducted involving patients with metastatic stage IV melanoma who received first-line treatment with anti-CTL4 and/or anti-PD-1 agents. The patients were categorized into two groups on the basis of their 1-year overall survival (OS): those with good outcomes (long-term OS ≥ 1 year) and those with poor outcomes (short-term OS < 1 year). Peripheral metabolomics was performed using baseline sera from 132 patients via 600 MHz Nuclear Magnetic Resonance (NMR) spectroscopy. Enriched functional analysis was conducted to identify the metabolic pathways in which significant metabolites were involved.
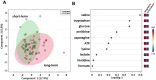
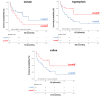
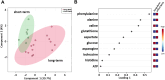
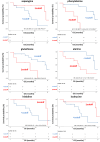
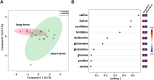
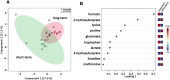
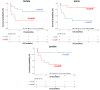
Results: Sparse partial least squares discriminant analysis (sPLS-DA) and loading plots obtained by analyzing the metabolomics profiles of samples collected before ICI treatment revealed significantly different levels of metabolites between the two groups (long-term OS vs. short-term OS). Specifically, lactate, tryptophan and valine significantly predicted the OS of the whole study population subjected to ICI immunotherapy; alanine, asparagine, glutathione, histidine, isoleucine and phenylalanine significantly predicted the OS of patients treated with ipilimumab; glucose, glutamine, histidine and proline significantly predicted the OS of patients treated with nivolumab; and lactate, lysine and proline significantly predicted the OS of patients treated with ipilimumab plus nivolumab. Notably, tryptophan levels were correlated with treatment response in the overall patient group, whereas histidine and lactate levels were associated with response in patients treated with ipilimumab and with ipilimumab plus nivolumab, respectively. Interestingly, higher pretreatment levels of histidine were commonly found in long-term OS subgroups of patients treated with ipilimumab, nivolumab or ipilimumab plus nivolumab. Interestingly, considering only those metabolites that predict OS after univariate analysis, higher histidine, and lower lactate and proline levels resulted as associated with favorable OS in at least two patient cohorts.
Conclusions: Overall, this exploratory liquid biopsy study revealed a strong correlation between the pretreatment levels of some metabolites and the OS of patients with metastatic stage IV melanoma treated with anti-CTL4 and/or anti-PD-1 antibodies in the first-line setting and revealed the potential of these molecules to predict outcomes and define personalized management and treatment strategies.
Keywords: Immune checkpoint inhibitors; Melanoma; Metabolomics; Serum biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Istituto Nazionale Tumori IRCCS Fondazione Pascale (protocol code: 33/17 OSS, date of approval: 10/1/2018). Informed consent was obtained from all subjects involved in the study. Consent for publication: Not applicable. Competing interests: The authors declare the following competing interests: P.A.A.: Stock and Other Ownership Interests: PrimeVax. Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis Biotech, Lunaphore Technologies, Seattle Genetics, ITeos Therapeutics, Medicenna, Bio-Al Health, ValoTx. Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), Sanofi (Inst), Pfizer (Inst). Travel, Accommodations, Expenses: Merck Sharp & Dohme, Pfizer.
Figures

References
-
- Luther C, Swami U, Zhang J, Milhem M, Zakharia Y. Advanced stage melanoma therapies: detailing the present and exploring the future. Crit Rev Oncol Hematol. 2019;133:99–111. - PubMed
-
- Long GV, Carlino MS, McNeil C, Ribas A, Gaudy-Marqueste C, Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. 2024;35(12):1191–9. - PubMed
-
- Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, Schadendorf D, Wagstaff J, et al. Final, 10-Year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2025;392(1):11–22. - PubMed
-
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

