Development and characterization of a novel immortalized human vaginal fibroblast cell line for advanced applications in reproductive health
- PMID: 40211367
- PMCID: PMC11984242
- DOI: 10.1186/s12958-025-01393-0
Development and characterization of a novel immortalized human vaginal fibroblast cell line for advanced applications in reproductive health
Abstract
Background: Reproductive health issues related to the vagina, face significant challenges due to the lack of standardized research models. Vaginal fibroblasts, which constitute approximately 55% of the vaginal wall's cellular composition, are crucial for tissue repair, remodelling, and reproductive health. These fibroblasts have broad applications in regenerative medicine and gynaecological treatments. Despite their importance, current research relies primarily on epithelial cells or primary vaginal fibroblasts, but primary fibroblasts are limited by their short lifespan, donor-to-donor variability, and susceptibility to senescence. Immortalized fibroblast lines offer a solution by extending the lifespan and enabling reproducible studies. However, a well-characterized immortalized human vaginal fibroblast line has not been established, highlighting the need for novel models to better understand and address vaginal-associated conditions.
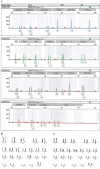
Methods: Primary human vaginal fibroblasts were immortalized via the lentiviral transfection of human telomerase reverse transcriptase. The resulting cell line was characterized through histological, immunofluorescent, RT-qPCR and flow cytometry analyses. Proliferation, senescence, gene expression, hormone responsiveness and genomic stability were assessed via quantitative polymerase chain reaction, transcriptome sequencing, gene set enrichment analysis, short tandem repeat profiling, and karyotype analysis.
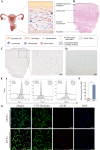
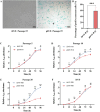
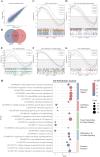
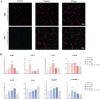
Results: The immortalized human vaginal fibroblasts (ihVFs) retained typical spindle-shaped fibroblast morphology and fibroblast-specific marker expression. Compared with primary vaginal fibroblasts, ihVF exhibited significantly reduced senescence, maintained sustained growth through extended culture passages, and preserved genetic stability. Transcriptome sequencing revealed high gene expression similarity between immortalized and primary fibroblasts, with no significant alterations in oncogenic pathways. PCR and immunofluorescent analyses revealed that ihVFs are responsive to estrogen and progesterone stimulation. Short tandem repeat analysis confirmed the novelty of the immortalized cell line, with no overlap with existing cell databases.
Conclusions: The novel ihVF cell line retains key phenotypic, functional, and genetic characteristics of primary vaginal fibroblasts, providing a stable, reproducible, and physiologically relevant model for reproductive health research. This cell line addresses the limitations of primary fibroblasts and has broad applications in tissue engineering, gynaecological disorder research, and drug screening, advancing our understanding of vaginal fibroblast biology and therapeutic interventions.
Keywords: Immortalized cell line; Reproductive health; Tissue engineering; Vaginal fibroblasts; hTERT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University (Approval No. 2024–124). Informed consent was obtained from all participants. Consent for publication: Informed consent for publication was obtained and signed by all participants involved in the study. All listed authors have reviewed this manuscript and have given their approval for its publication. Competing interests: The authors declare no competing interests.
Figures


References
-
- Boehnke K, Mirancea N, Pavesio A, Fusenig NE, Boukamp P, Stark H-J. Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol. 2007;86:731–46 - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFC2701400/National Key Research and Development Program of China
- 2021YFC2701400/National Key Research and Development Program of China
- 2021YFC2701400/National Key Research and Development Program of China
- 2021YFC2701400/National Key Research and Development Program of China
- 2021YFC2701400/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources

