Extracellular matrix stiffness: mechanisms in tumor progression and therapeutic potential in cancer
- PMID: 40211368
- PMCID: PMC11984264
- DOI: 10.1186/s40164-025-00647-2
Extracellular matrix stiffness: mechanisms in tumor progression and therapeutic potential in cancer
Abstract
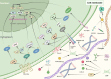
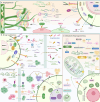
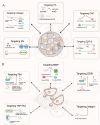
Tumor microenvironment (TME) is a complex ecosystem composed of both cellular and non-cellular components that surround tumor tissue. The extracellular matrix (ECM) is a key component of the TME, performing multiple essential functions by providing mechanical support, shaping the TME, regulating metabolism and signaling, and modulating immune responses, all of which profoundly influence cell behavior. The quantity and cross-linking status of stromal components are primary determinants of tissue stiffness. During tumor development, ECM stiffness not only serves as a barrier to hinder drug delivery but also promotes cancer progression by inducing mechanical stimulation that activates cell membrane receptors and mechanical sensors. Thus, a comprehensive understanding of how ECM stiffness regulates tumor progression is crucial for identifying potential therapeutic targets for cancer. This review examines the effects of ECM stiffness on tumor progression, encompassing proliferation, migration, metastasis, drug resistance, angiogenesis, epithelial-mesenchymal transition (EMT), immune evasion, stemness, metabolic reprogramming, and genomic stability. Finally, we explore therapeutic strategies that target ECM stiffness and their implications for tumor progression.
Keywords: Cancer therapy; Extracellular matrix; Mechanical sensor; Stiffness; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors read and approved the final manuscript for publication. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

