Critical roles of IL-6 signaling in myoblast differentiation of human adipose-derived mesenchymal stem cells
- PMID: 40211386
- PMCID: PMC11983861
- DOI: 10.1186/s41232-025-00373-6
Critical roles of IL-6 signaling in myoblast differentiation of human adipose-derived mesenchymal stem cells
Abstract
Background: Ectopic fat is also formed in muscles as well as the liver, where adipose-derived mesenchymal stem cells (ADSCs) promote adipogenesis. On the other hand, after muscle injury, muscle satellite cells (SCs) contribute to muscle repair through myodifferentiation. Human ADSCs are multipotent stem cells, but it remains unclear whether they are involved in myoblast differentiation. The aim is to find a novel myogenic cytokine and its signaling pathway that promotes the differentiation of human ADSCs-a potential source of new muscle precursor cells-into myoblasts.
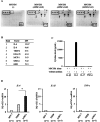
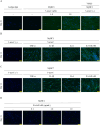
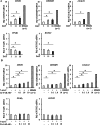
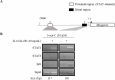
Methods: An array kit was used to detect cytokines produced by ADSCs. After treating ADSCs with the DNA methyltransferase inhibitor 5-Aza-2'-deoxycytidine (5-aza-C) and different JAK inhibitors, MyHC1, a myodifferentiation marker, was detected by immunofluorescence staining and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The expression status of signaling molecules was determined by Western blotting and the recruitment of transcription factors to the MYOG promoter by chromatin immunoprecipitation (ChIP).
Results: IL-6 was detected at high concentrations in the culture supernatant of ADSCs. ADSCs stimulated with 5-aza-C became strongly positive for MyHC1 on day 21 post-stimulation. When co-stimulated with 5-aza-C and IL-6/sIL-6R, ADSCs became positive for MyHC1 protein and upregulated MYOG mRNA as early as day 14 post-stimulation. Co-stimulation with 5-aza-C and IL-6/sIL-6R resulted in phosphorylation of STAT1 and STAT3. The addition of a JAK2 inhibitor, but not JAK1/3 inhibitors, abolished the MyHC1 positivity and phosphorylation of STAT1 and STAT3. Co-stimulation with 5-aza-C and IL-6/sIL-6R during the myogenesis process resulted in the recruitment of STAT1, but not STAT3, to the MYOG promoter. Myoblast differentiation induced by stimulation with 5-aza-C was enhanced by activation of the IL-6/JAK2/STAT1/MYOG pathway.
Conclusions: Therefore, sustained IL-6/JAK2/STAT1 activation may serve as an important driver of human ADSC differentiation into myoblast, suggesting an important candidate signaling pathway for ameliorating muscle atrophy.
Keywords: 5-aza-C; ADSCs; IL-6; Myoblast; STAT1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: S. Nakayamada has received consulting fees, speaking fees, lecture fees, and/or honoraria from AstraZeneca, GlaxoSmithKline, Pfizer, Bristol-Myers, Astellas, Asahi-kasei, AbbVie, Chugai, Sanofi, Eisai, Gilead Sciences, Mitsubishi-Tanabe, Janssen, Eli Lilly, Boehringer Ingelheim, and Ayumi. Y. Okada has received lecture fees from AstraZeneca, MSD, Ono, Mitsubishi-Tanabe, Bayer, Novo Nordisk, Eli Lilly, and Boehringer Ingelheim and research funds from Kowa and Mitsubishi-Tanabe. Y. Tanaka has received speaking fees and/or honoraria from Abbvie, Eisai, Chugai, Eli-Lilly, Behringer-Ingelheim, GlaxoSmithKline, Taisho, AstraZeneca, Daiichi-Sankyo, Gilead, Pfizer, UCB, Asahi-kasei, and Astellas and received research grants from Behringer-Ingelheim, Taisho, and Chugai. The other authors declare that they have no competing interests.
Figures


References
-
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. 10.1093/jn/127.5.990S. - PubMed
-
- Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–7. 10.1046/j.1467-789x.2001.00031.x. - PubMed
-
- Olmos Martínez JM, Hernández Martínez P, González Macías J. Frailty, sarcopenia and osteoporosis. Med Clin (Barc). 2024;163(2):e17–23. 10.1016/j.medcli.2024.03.004. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

