Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study
- PMID: 40211417
- PMCID: PMC11987368
- DOI: 10.1186/s13075-025-03528-5
Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study
Abstract
Background: Upadacitinib (UPA), an oral Janus kinase inhibitor, has shown efficacy with an acceptable safety profile in rheumatoid arthritis (RA) clinical trials.
Objective: To assess the real-world effectiveness and safety of UPA in adults with moderate-to-severe RA in the UPHOLD observational study.
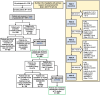
Methods: Co-primary endpoints were: (i) proportion of patients achieving disease activity score in 28 joints using C-reactive protein (DAS28[CRP]) remission (< 2.6) at 6 months; and (ii) proportion of those patients maintaining remission at 12 months. Additional analyses included proportions of patients achieving and maintaining DAS28(CRP) low disease activity (LDA; ≤ 3.2), other composite measures of disease activity, and subgroup analyses by therapy strategy and prior treatment. Treatment-emergent adverse events (TEAEs) in the full analysis set (FAS; patients receiving ≥ 1 UPA dose) were reported through August 10, 2023. Co-primary and selected secondary endpoints were analyzed by modified non-responder imputation (mNRI) in modified (m)FAS1 (FAS patients who completed 6 months of treatment and had DAS28[CRP] data available, and those who discontinued before 6 months) and mFAS2 (mFAS1 patients who achieved remission at 6 months, completed 12 months of treatment, and had DAS28[CRP] data available, and those who discontinued between 6 and 12 months); and as observed (AO) in patients with non-missing data.
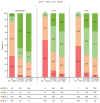
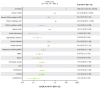
Results: Of 1719 participants, 1717 were enrolled; 1701 comprised the FAS. Overall, 400/1719 (23.3%) patients discontinued before 12 months. Of mFAS1 patients, 499 (mNRI: 499/1074 [46.5%]; AO: 499/902 [55.3%]) achieved DAS28(CRP) remission at 6 months; of mFAS2 patients, 269 (mNRI: 269/340 [79.1%]; AO: 269/317 [84.9%]) maintained remission at 12 months. DAS28(CRP) remission or LDA rates were consistent regardless of whether UPA was initiated and maintained as monotherapy or combination therapy. Similar responses were observed across prior treatment subgroups. Among selected TEAEs of special interest, herpes zoster and serious infection occurred at 3.12 and 2.62 events/100 patient-years, respectively. No new safety signals were identified.
Conclusions: UPA demonstrated real-world effectiveness in moderate-to-severe RA, with approximately half of patients achieving DAS28(CRP) remission at 6 months and most maintaining remission through 12 months. The real-world benefit-risk profile of UPA remains favorable and is consistent with phase 3 clinical trial data.
Trial registration: NCT04497597.
Keywords: Effectiveness; Real-world; Rheumatoid arthritis; Safety; Upadacitinib.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the International Council on Harmonisation guidelines and the Declaration of Helsinki. The trial protocol was approved by independent ethics committees and institutional review boards. Written informed consent was provided by patients ahead of study screening. Consent for publication: Not applicable. Competing interests: AO has served as a consultant and/or on advisory boards and/or undertaken clinical trials for AbbVie, GSK, Janssen, Lilly, Novartis, and Pfizer. EF has received honoraria and research grants from AbbVie, BMS, Galapagos, Lilly, MSD, Novartis, Pfizer, Roche, and Sobi. PS has received honoraria and research grants from AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, and UCB through the University of Crete Special Account for Research. JA has received honoraria from AbbVie, AstraZeneca, Biogen, BMS, Fresenius Kabi, Galapagos, Janssen, Lilly, Medac, MSD, Nordic Pharma, Novartis, Pfizer, Roche-Chugai, Sandoz, and Sanofi; and research grants from BMS, Fresenius Kabi, Galapagos, Novartis (Dreamer), and Pfizer (Passerelle). MR has received honoraria and/or support to participate in academic events from AbbVie, Bayer, Pfizer, and Roche. RN has no conflicts of interest to declare. EMB, TG, ILG, and SS are AbbVie employees and may own AbbVie stock or options. DZ has received speaker fees and/or advisory honoraria from AbbVie, AstraZeneca, GSK, Janssen, Lilly, Novartis, Pfizer, Roche, and Sandoz, and research grants from Pfizer. SA has received speaker fees, research grants, and advisory honoraria from AbbVie, Amgen, AstraZeneca, BMS, Gilead, GSK, Hikma, Janssen, Lilly, Novartis, Organon, Pfizer, Roche, Sandoz, Sanofi, and Takeda.
Figures

References
-
- Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Mod Rheumatol. 2020;30:779–87. - PubMed
-
- Winthrop KL, Strand V, van der Heijde DM, Mease PJ, Crow MK, Weinblatt M, et al. The unmet need in rheumatology: reports from the targeted therapies meeting 2016. Clin Exp Rheumatol. 2016;34:69–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

