Gaucher disease type 3 from infancy through adulthood: a conceptual model of signs, symptoms, and impacts associated with ataxia and cognitive impairment
- PMID: 40211441
- PMCID: PMC11987173
- DOI: 10.1186/s13023-025-03654-y
Gaucher disease type 3 from infancy through adulthood: a conceptual model of signs, symptoms, and impacts associated with ataxia and cognitive impairment
Abstract
Background: Gaucher disease type 3 (GD3) is a lysosomal storage disease characterized by diverse neurological and systemic manifestations. Symptoms of ataxia, cognitive impairment, and other systemic symptoms profoundly impact daily activities and the quality of life for individuals living with the disease. Development of a conceptual model of disease for persons living with GD3 from birth to adulthood would enable objective monitoring of disease progression and assessment of treatment benefits.
Methods: A targeted literature review, interviews with clinical experts, and interviews with individuals and their caregivers living in the UK and the US were carried out to understand the patient experience. Interviews were transcribed and de-identified data were analyzed to identify signs, symptoms, and impacts of ataxia, cognitive impairment, and other systemic impairments. A conceptual model was developed by integrating relevant signs, symptoms, and impacts experienced from birth through adulthood.
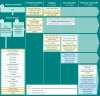
Results: Review of symptoms and impacts of GD3 from three published scientific articles, and interviews with six clinical experts, 12 individuals living with GD3, and 12 caregivers, identified 58 patient experience concepts associated with GD3. Signs and symptoms associated with ataxia appear during the first 3 years of life and persist beyond 5 years of age, while signs and symptoms related to neurocognition appear later in life. Difficulty in shifting gaze and/or tracking objects, ataxia, tremors, memory problems, difficulty in processing new information, fatigue, and bone pain are most salient concepts for GD3. In patients aged ≤ 5 years, motor manifestations and symptoms were far more prevalent than neurocognitive signs and symptoms. Inability to work or perform at school, limited social and family engagements, restricted mobility (walking, driving, public transportation), and declining independence were the most important impacts on individuals with GD3.
Conclusions: Heterogeneity exists in GD3 manifestations, especially neuromuscular and neurocognitive signs, symptoms, and impacts, across all age ranges of individuals living with GD3. The conceptual model developed in the study provided a comprehensive understanding of the disease in individuals with GD3.
Keywords: Ataxia; Cognitive impairment; Conceptual model; GD3; Gaucher disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was reviewed and approved by Advarra IRB (approved on 29 Nov2018) and informed consent was collected from patients and caregivers who participated in the study. The study was conducted in accordance with the protocol, applicable regulations and guidelines governing clinical study conduct, and the ethics principles given in the Declaration of Helsinki. De-identified patient data were collected in compliance with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Consent for publication: Patients and caregivers provided informed consent to publish data collected during the interviews in scientific journals. Competing interests: Raphael Schiffman has no competing interests to disclose. Alaa Hamed and Isabela Batsu are employees of Sanofi. Ruth Pulikottil-Jacob and Walter Heine were employees of Sanofi at the time of study conduct. James Turnbull and Robert Krupnick are employees of IQVIA, Inc. Chad Gwaltney is an employee of Gwaltney Consulting and was a paid consultant of Sanofi. Walter Heine was an employee of Sanofi at the time of study conduct. Eugen Mengel has received research grants, speakers’ honoraria, and consultation fees from Alexion, Amicus, Avrobio, Cyclo Therapeutics, Idorsia, Orphazyme, Sanofi, and Takeda.
Figures
References
-
- Nalysnyk L, Rotella P, Simeone JC, Hamed A, Weinreb N. Gaucher disease epidemiology and natural history: a comprehensive review of the literature. Hematology. 2017;22(2):65–73. 10.1080/10245332.2016.1240391. - PubMed
-
- Schiffmann R, Mengel E, Wallace M, Rochmann C, Turnbull J, Krupnick R, et al. Qualitative study of the patient experience with venglustat for Gaucher disease type 3 in a phase 2 open-Label, Multicenter, Multinational Study (LEAP). Adv Ther. 2024;41(7):2907–23. 10.1007/s12325-024-02881-2. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

