The chromosomal challenge of human embryos: Mechanisms and fundamentals
- PMID: 40211536
- PMCID: PMC12050003
- DOI: 10.1016/j.xhgg.2025.100437
The chromosomal challenge of human embryos: Mechanisms and fundamentals
Abstract
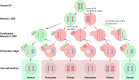
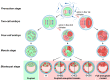
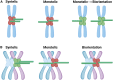
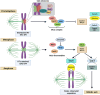
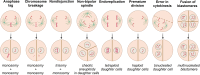
Chromosomal abnormalities in human pre-implantation embryos, originating from either meiotic or mitotic errors, present a significant challenge in reproductive biology. Complete aneuploidy is primarily linked to errors during the resumption of meiosis in oocyte maturation, which increase with maternal age, while mosaic aneuploidies result from mitotic errors after fertilization. The biological causes of these abnormalities are increasingly becoming a topic of interest for research groups and clinical specialists. This review explores the intricate processes of meiotic and early mitotic divisions in embryos, shedding light on the mechanisms that lead to changes in chromosome number in daughter cells. Key factors in meiotic division include difficulties in spindle assembly without centrosomes, kinetochore (KT) orientation disturbances, and inefficient cell-cycle checkpoints. The weakening of cohesion molecules that bind chromosomes, exacerbated by maternal aging, further complicates chromosomal segregation. Mitotic errors in early development are influenced by defects in sperm centrosomes, KT misalignment, and the gradual depletion of maternal regulatory factors. Coupled with the inactive or partially active embryonic genome, this depletion increases the likelihood of chromosomal aberrations. While various theoretical mechanisms for these abnormalities exist, current data remain insufficient to determine their exact contributions. Continued research is essential to unravel these complex processes and improve outcomes in assisted reproductive technologies.
Keywords: aneuploidy; cell-cycle checkpoints; chromosomal mosaicism; chromosome segregation errors; kinetochore orientation; meiotic errors; mitotic errors.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Current quantitative methodologies for preimplantation genetic testing frequently misclassify meiotic aneuploidies as mosaic.Fertil Steril. 2025 Aug;124(2):307-318. doi: 10.1016/j.fertnstert.2025.02.018. Epub 2025 Feb 15. Fertil Steril. 2025. PMID: 39961387
-
Approximate Bayesian computation supports a high incidence of chromosomal mosaicism in blastocyst-stage human embryos.bioRxiv [Preprint]. 2024 Dec 2:2024.11.26.625484. doi: 10.1101/2024.11.26.625484. bioRxiv. 2024. Update in: Genetics. 2025 Aug 01:iyaf149. doi: 10.1093/genetics/iyaf149. PMID: 39677623 Free PMC article. Updated. Preprint.
-
Approximate Bayesian computation supports a high incidence of chromosomal mosaicism in blastocyst-stage human embryos.Genetics. 2025 Aug 1:iyaf149. doi: 10.1093/genetics/iyaf149. Online ahead of print. Genetics. 2025. PMID: 40746180
-
Non-homologous sequence interactions during meiosis: meiotic challenges and evolutionary opportunities.Curr Opin Genet Dev. 2025 Aug;93:102365. doi: 10.1016/j.gde.2025.102365. Epub 2025 May 23. Curr Opin Genet Dev. 2025. PMID: 40409127 Review.
-
Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection.Cochrane Database Syst Rev. 2006 Jan 25;(1):CD005291. doi: 10.1002/14651858.CD005291.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2020 Sep 8;9:CD005291. doi: 10.1002/14651858.CD005291.pub3. PMID: 16437524 Updated.
References
-
- Franasiak J.M., Forman E.J., Hong K.H., Werner M.D., Upham K.M., Treff N.R., Scott R.T. Aneuploidy across individual chromosomes at the embryonic level in trophectoderm biopsies: changes with patient age and chromosome structure. J. Assist. Reprod. Genet. 2014;31:1501–1509. doi: 10.1007/s10815-014-0333-x. - DOI - PMC - PubMed
-
- Volodyaev I., Ivanova A., Korchivaia E., Surnov A., Pomerantseva E., Lebedev I.N., Semenova M.L., Mazunin I. The chromosomal challenge of human embryos: prevalence of aneuploidy and mosaicism. F&S Reviews. 2025;6 doi: 10.1016/j.xfnr.2024.100082. - DOI
-
- Colorado Center for Reproductive Medicine (2017). CCRM Reports the Lowest Chromosomal Mosaicism Rates in the U.S. At Less than Three Percent across the CCRM Network. In J. Peel, ed.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

