Ovarian Function Restoration with Biomimetic Scaffold Incorporating Angiogenic Molecules and Antioxidant in Chemotherapy-Induced Perimenopausal Model
- PMID: 40211585
- PMCID: PMC12083436
- DOI: 10.1002/adhm.202403944
Ovarian Function Restoration with Biomimetic Scaffold Incorporating Angiogenic Molecules and Antioxidant in Chemotherapy-Induced Perimenopausal Model
Abstract
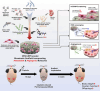
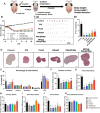
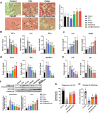
Chemotherapy-induced premature ovarian insufficiency (POI) is a major cause of infertility and hormonal imbalance in young female cancer survivors. In this study, developed a biomimetic scaffold is developed that incorporates polydeoxyribonucleotide (PDRN) and melatonin to restore ovarian function. The scaffold is designed to mimic the ovarian extracellular matrix (ECM), enhancing angiogenesis, promoting antioxidant effects, and reducing reactive oxygen species (ROS). Human embryonic stem cell-derived mesenchymal progenitor cells (hESC-MPCs) are also incorporated to further support tissue regeneration. The scaffold demonstrated strong efficacy in improving cell survival, promoting folliculogenesis, and restoring ovarian function in a chemotherapy-induced perimenopausal mouse model. Results showed that the scaffold enhanced vascularization, reduced fibrosis, and normalized hormone levels, including estrogen (E2) and anti-Müllerian hormone (Amh). Additionally, the transplantation of the scaffold restored fertility rates and increased the number of offspring in treated mice. This approach presents a promising solution for improving ovarian recovery and fertility preservation in patients with chemotherapy-induced POI, offering a novel therapeutic strategy for reproductive health.
Keywords: ROS scavenging; angiogenesis; anti‐inflammation; biomimetic scaffold; chemotherapy‐induced premature ovarian insufficiency; embryonic stem cell‐derived mesenchymal progenitor cells; ovarian function restoration.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Ke H., Tang S., Guo T., Hou D., Jiao X., Li S., Luo W., Xu B., Zhao S., Li G., Zhang X., Xu S., Wang L., Wu Y., Wang J., Zhang F., Qin Y., Jin L., Chen Z.‐J., Nat. Med. 2023, 29, 483; - PMC - PubMed
- b) Lie Fong S., Lugtenburg P. J., Schipper I., Themmen A. P. N., de Jong F. H., Sonneveld P., Laven J. S. E., Hum. Reprod. 2008, 23, 674. - PubMed
-
- a) Perin E. C., Borow K. M., Henry T. D., Mendelsohn F. O., Miller L. W., Swiggum E., Adler E. D., Chang D. H., Fish R. D., Bouchard A., Jenkins M., Yaroshinsky A., Hayes J., Rutman O., James C. W., Rose E., Itescu S., Greenberg B., J. Am. Coll. Cardiol. 2023, 81, 849; - PubMed
- b) Amirdelfan K., Bae H., McJunkin T., DePalma M., Kim K., Beckworth W. J., Ghiselli G., Bainbridge J. S., Dryer R., Deer T. R., Brown R. D., The Spine Journal 2021, 21, 212. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

