FREQ-NESS Reveals the Dynamic Reconfiguration of Frequency-Resolved Brain Networks During Auditory Stimulation
- PMID: 40211612
- PMCID: PMC12120751
- DOI: 10.1002/advs.202413195
FREQ-NESS Reveals the Dynamic Reconfiguration of Frequency-Resolved Brain Networks During Auditory Stimulation
Abstract
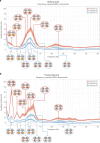
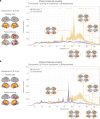
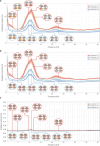
The brain is a dynamic system whose network organization is often studied by focusing on specific frequency bands or anatomical regions, leading to fragmented insights, or by employing complex and elaborate methods that hinder straightforward interpretations. To address this issue, a new analytical pipeline named FREQuency-resolved Network Estimation via Source Separation (FREQ-NESS) is introduced. This pipeline is designed to estimate the activation and spatial configuration of simultaneous brain networks across frequencies by analyzing the frequency-resolved multivariate covariance between whole-brain voxel time series. In this study, FREQ-NESS is applied to source-reconstructed magnetoencephalography (MEG) data during resting state and isochronous auditory stimulation. Our results reveal simultaneous, frequency-specific brain networks during resting state, such as the default mode, alpha-band, and motor-beta networks. During auditory stimulation, FREQ-NESS detects: 1) emergence of networks attuned to the stimulation frequency, 2) spatial reorganization of existing networks, such as alpha-band networks shifting from occipital to sensorimotor areas, 3) stability of networks unaffected by auditory stimuli. Furthermore, auditory stimulation significantly enhances cross-frequency coupling, with the phase of auditory networks attuned to the stimulation modulating gamma band amplitude in medial temporal lobe networks. In conclusion, FREQ-NESS effectively maps the brain's spatiotemporal dynamics, providing a comprehensive view of brain function by revealing a landscape of simultaneous, frequency-resolved networks and their interaction.
Keywords: auditory processing; brain networks; cross‐frequency coupling (CFC); generalized eigendecomposition (GED); magnetoencephalography (MEG); resting state.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Neuromagnetic activation and oscillatory dynamics of stimulus-locked processing during naturalistic viewing.Neuroimage. 2020 Aug 1;216:116414. doi: 10.1016/j.neuroimage.2019.116414. Epub 2019 Nov 30. Neuroimage. 2020. PMID: 31794854
-
Fast reconfiguration of high-frequency brain networks in response to surprising changes in auditory input.J Neurophysiol. 2012 Mar;107(5):1421-30. doi: 10.1152/jn.00817.2011. Epub 2011 Dec 14. J Neurophysiol. 2012. PMID: 22170972 Free PMC article. Clinical Trial.
-
Human Auditory-Motor Networks Show Frequency-Specific Phase-Based Coupling in Resting-State MEG.Hum Brain Mapp. 2025 Jan;46(1):e70045. doi: 10.1002/hbm.70045. Hum Brain Mapp. 2025. PMID: 39757971 Free PMC article.
-
Group-level spatial independent component analysis of Fourier envelopes of resting-state MEG data.Neuroimage. 2014 Feb 1;86:480-91. doi: 10.1016/j.neuroimage.2013.10.032. Epub 2013 Oct 31. Neuroimage. 2014. PMID: 24185028
-
Emotion brain network topology in healthy subjects following passive listening to different auditory stimuli.PeerJ. 2024 Jul 19;12:e17721. doi: 10.7717/peerj.17721. eCollection 2024. PeerJ. 2024. PMID: 39040935 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
