Molecular Tools to Study and Control Dopaminergic Neurotransmission With Light
- PMID: 40211663
- PMCID: PMC12322515
- DOI: 10.1002/med.22112
Molecular Tools to Study and Control Dopaminergic Neurotransmission With Light
Abstract
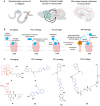
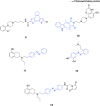
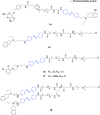
Dopaminergic neurotransmission is involved in several important brain functions, such as motor control, learning, reward-motivated behavior, and emotions. Dysfunctions of dopaminergic system may lead to the development of various neurological and psychiatric disorders, like Parkinson's disease, schizophrenia, depression, and addictions. Despite years of sustained research, it is not fully established how dopaminergic neurotransmission governs these important functions through a relatively small number of neurons that release dopamine. Light-driven neurotechnologies, based on the use of small light-regulated molecules or overexpression of light-regulated proteins in neurons, have greatly contributed to the advancement of our understanding of dopaminergic circuits and our ability to control them selectively. Here, we overview the current state-of-the-art of light-driven control of dopaminergic neurotransmission. While we provide a concise guideline for the readers interested in pharmacological, pharmacogenetic, and optogenetic approaches to modulate dopaminergic neurotransmission, our primary focus is on the usage of photocaged and photo-switchable small dopaminergic molecules. We argue that photopharmacology, photoswitchable molecules of varied modalities, can be employed in a wide range of experimental paradigms, providing unprecedent insights into the principles of dopaminergic control, and represent the most promising light-based therapeutic approach for spatiotemporally precise correction of dopamine-related neural functions and pathologies.
Keywords: azobenzene; caged ligands; catecholamine; dopamine; neuromodulation; neuronal circuits; optogenetics; optopharmacology; photoisomerization; photopharmacology; photoswitch.
© 2025 The Author(s). Medicinal Research Reviews published by Wiley Periodicals LLC.
Figures

References
-
- Beaulieu J.‐M. and Gainetdinov R. R., “The Physiology, Signaling, and Pharmacology of Dopamine Receptors,” Pharmacological Reviews 63 (2011): 182–217. - PubMed
-
- Missale C., Nash S. R., Robinson S. W., Jaber M., and Caron M. G., “Dopamine Receptors: From Structure to Function,” Physiological Reviews 78 (1998): 189–225. - PubMed
-
- Jaber M., Robinson S. W., Missale C., and Caron M. G., “Dopamine Receptors and Brain Function,” Neuropharmacology 35 (1996): 1503–1519. - PubMed

