Palivizumab coverage rates among moderate-to-late preterm infants in Korea: a nationwide cross-sectional study
- PMID: 40211817
- PMCID: PMC12178765
- DOI: 10.4178/epih.e2025015
Palivizumab coverage rates among moderate-to-late preterm infants in Korea: a nationwide cross-sectional study
Abstract
Objectives: Since October 2016, Korea has implemented a national reimbursement program for palivizumab aimed at moderate-to-late preterm (MLPT) infants born between 32 0/7 weels and 35 6/7 weeks of gestation during the respiratory syncytial virus (RSV) season (October-March). However, large-scale data on coverage rates and associated factors remain limited. This study evaluated palivizumab coverage rates and identified predictive factors influencing its administration in MLPT infants.
Methods: This nationwide, population-based cross-sectional study utilized data from the Korean National Health Insurance Service collected between October 2016 and March 2019. MLPT infants eligible for palivizumab reimbursement were divided into administration and non-administration groups. Seasonal and overall coverage rates were assessed. A multivariate logistic regression analysis examined factors associated with palivizumab administration, with a focus on infant and maternal characteristics.
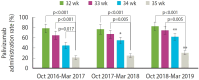
Results: Among 2,843 eligible MLPT infants, 1,201 (42.2%) received palivizumab, while 1,642 (57.8%) did not. Although coverage rates increased annually, they remained suboptimal. Lower palivizumab prophylaxis coverage was observed in infants with higher gestational ages, female sex, absence of low birth weight, those born in March, residents of non-capital areas, infants not admitted to a neonatal intensive care unit at birth, and infants of mothers aged <35 years.
Conclusions: In the initial 3 RSV seasons following the introduction of palivizumab reimbursement for MLPT infants in Korea, the overall coverage rate was low (42.2%). National policies targeting infants with higher gestational ages, those born in March, and those residing in non-capital areas are necessary to improve coverage and ensure equitable RSV prophylaxis.
Keywords: Palivizumab; Premature infant; Prophylaxis; Respiratory syncytial virus; Respiratory tract infections; Vaccination coverage.
Conflict of interest statement
The authors have no conflicts of interest to declare for this study.
Figures


Similar articles
-
Impact of Palivizumab in Preventing Severe Acute Lower Respiratory Infection in Moderate-to-Late Preterm Infants: A Nationwide Cohort Study.J Korean Med Sci. 2024 Nov 11;39(43):e279. doi: 10.3346/jkms.2024.39.e279. J Korean Med Sci. 2024. PMID: 39536788 Free PMC article.
-
Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis.Pediatr Crit Care Med. 2011 Sep;12(5):580-8. doi: 10.1097/PCC.0b013e3182070990. Pediatr Crit Care Med. 2011. PMID: 21200358
-
Impact of respiratory tract infections on spinal muscular atrophy with focus on respiratory syncytial virus infections: a single-centre cohort study.Swiss Med Wkly. 2024 Oct 1;154:3573. doi: 10.57187/s.3573. Swiss Med Wkly. 2024. PMID: 39463279
-
Systematic Review of the Safety and Efficacy of Palivizumab among Infants and Young Children with Cystic Fibrosis.Pharmacotherapy. 2017 Jun;37(6):755-769. doi: 10.1002/phar.1936. Epub 2017 May 29. Pharmacotherapy. 2017. PMID: 28423192
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

