The Heart Has Intrinsic Ketogenic Capacity that Mediates NAD+ Therapy in HFpEF
- PMID: 40211954
- PMCID: PMC12063684
- DOI: 10.1161/CIRCRESAHA.124.325550
The Heart Has Intrinsic Ketogenic Capacity that Mediates NAD+ Therapy in HFpEF
Abstract
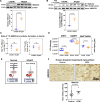
Background: Heart failure with preserved ejection fraction (HFpEF) has overtaken heart failure with reduced ejection fraction as the leading type of heart failure globally and is marked by high morbidity and mortality rates, yet with only a single approved pharmacotherapy: SGLT2i (sodium-glucose co-transporter 2 inhibitor). A prevailing theory for the mechanism underlying SGLT2i is nutrient deprivation signaling, of which ketogenesis is a hallmark. However, it is unclear whether the canonical ketogenic enzyme, HMGCS2 (3-hydroxy-3-methylglutaryl-coenzyme A synthase 2), plays any cardiac role in HFpEF pathogenesis or therapeutic response.
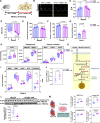
Methods: We used human myocardium, human HFpEF and heart failure with reduced ejection fraction transcardiac blood sampling, an established murine model of HFpEF, ex vivo Langendorff perfusion, stable isotope tracing in isolated cardiomyocytes, targeted metabolomics, proteomics, lipidomics, and a novel cardiomyocyte-specific conditional HMGCS2-deficient model that we generated.
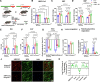
Results: We demonstrate, for the first time, the intrinsic capacity of the human heart to produce ketones via HMGCS2. We found that increased acetylation of HMGCS2 led to a decrease in the enzyme's specific activity. However, this was overcome by an increase in the steady-state levels of protein. Oxidized form of nicotinamide adenine dinucleotide repletion restored HMGCS2 function via deacetylation, increased fatty acid oxidation, and rescued cardiac function in HFpEF. Critically, using a conditional, cardiomyocyte-specific HMGCS2 knockdown murine model, we revealed that the oxidized form of nicotinamide adenine dinucleotide is unable to rescue HFpEF in the absence of cardiomyocyte HMGCS2.
Conclusions: The canonical ketogenic enzyme, HMGCS2, mediates the therapeutic effects of the oxidized form of nicotinamide adenine dinucleotide repletion in HFpEF by restoring normal lipid metabolism and mitochondrial function.
Keywords: heart failure; ketone bodies; myocardium; oxygen consumption; stroke volume.
Conflict of interest statement
None.
Figures


Comment in
-
Intrinsic Ketogenic Capacity of the Heart: Mechanisms and Therapeutic Potential.Circ Res. 2025 May 9;136(10):1131-1133. doi: 10.1161/CIRCRESAHA.125.326560. Epub 2025 May 8. Circ Res. 2025. PMID: 40339048 No abstract available.
References
-
- Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Müller MJ. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369–1377. doi: 10.3945/ajcn.2010.29885 - PMC - PubMed
-
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795 - PubMed
-
- Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG outcome trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330 - PubMed
-
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007 - PubMed
-
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

